Regulaci - PowerPoint PPT Presentation
1 / 11
Title:
Regulaci
Description:
Title: Slide 1 Author: Alfonso Last modified by: Alfonso Created Date: 12/18/2005 4:25:36 PM Document presentation format: On-screen Show Company: Indiana University – PowerPoint PPT presentation
Number of Views:89
Avg rating:3.0/5.0
Title: Regulaci
1
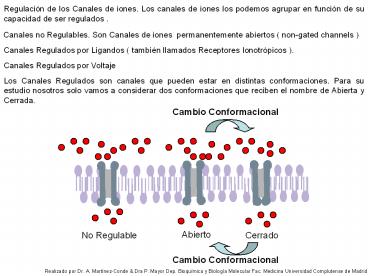
Regulación de los Canales de iones. Los canales
de iones los podemos agrupar en función de su
capacidad de ser regulados .
Canales no Regulables. Son Canales de iones
permanentemente abiertos ( non-gated channels )
Canales Regulados por Ligandos ( también llamados
Receptores Ionotrópicos ).
Canales Regulados por Voltaje
Los Canales Regulados son canales que pueden
estar en distintas conformaciones. Para su
estudio nosotros solo vamos a considerar dos
conformaciones que reciben el nombre de Abierta y
Cerrada.
Cambio Conformacional
Abierto
No Regulable
Cerrado
Cambio Conformacional
Realizado por Dr. A. Martínez-Conde Dra P.
Mayor Dep. Bioquímica y Biología Molecular Fac.
Medicina Universidad Complutense de Madrid
2
Realizado por Dr. A. Martínez-Conde Dra P.
Mayor Dep. Bioquímica y Biología Molecular Fac.
Medicina Universidad Complutense de Madrid
3
Realizado por Dr. A. Martínez-Conde Dra P.
Mayor Dep. Bioquímica y Biología Molecular Fac.
Medicina Universidad Complutense de Madrid
4
Canales Regulados por ligandos. Son Canales que
presentan diferentes conformaciones en presencia
o ausencia de Ligando.
Canal de iones que está Abierto en Reposo. Se
Cierra al desaparecer el Ligando. Un ejemplo
sería el Canal de Na y Ca de membrana de
Bastones Retinianos, sensible a cGMP
Cambio Conformacional
El Canal en la célula en Reposo se encuentra en
su conformación Abierta, La alta concentración
citosólica de cGMP hace que este Ligando esté
unido al Canal de Na y Ca y como consecuencia
se encuentre estabilizado en su forma Abierta.
La degradación del cGMP citosólico por el enzima
fosfodiesterasa, causa un descenso en su
concentración y como consecuencia una disociación
del Canal. La disociación causa un cambio
conformacional a la forma Cerrada.
Al cesar el estímulo, y volver la célula a su
estado de reposo, la concentración de cGMP se
recupera, uniendose de nuevo al sitio alostérico
del Canal. Pasando a la conformación Abierta, en
la que queda estabilizado hasta que se produzca
un nuevo estímulo.
Cerrado
Abierto
Cambio Conformacional
Realizado por Dr. A. Martínez-Conde Dra P.
Mayor Dep. Bioquímica y Biología Molecular Fac.
Medicina Universidad Complutense de Madrid
5
La estructura de un canal de K
Bolas rojas grandes agua / Bolas rojas
pequeñas grupos C0 cadena se señalan los
aminácidos / Bolas verdes K Close-up Only
three of the subunits are shown to provide a
clearer view of the pore. All seven of the
possible K locations are shown. However, only
four, at most, are occupied at the same time the
other sites contain waters. Residues 75-79
comprise the selectivity pore and are shown as
Sticks, colored CPK. The mainchain CO's of these
residues coordinate the K ions. The water-filled
cavity is colored cyan. All of the waters shown
here are highly-ordered and thus differ from the
"bulk" water surrounding the channel. K at
sites 2 4 Each K ion is coordinated by eight
oxygens with water between them. For example, K
at site 4 is coordinated by the side chains and
CO's of Thr 75. K between sites The K ions
are coordinated by only four of the mainchain
CO's. For example, the K between sites 2 and 3
is coordinated only by Val 76. A K is about to
enter the pore from the cavity. K at sites 1
3 The K ions are coordinated by eight mainchain
CO's. For example, K at site 1 is coordinated
by Gly77 and Tyr78. The pore is normally occupied
by two K ions and two waters. Concerted movement
of the two ions and their waters leads to K exit
from, or entry into the cell. The scripted
animation shows the sequence of steps in the exit
pathway.
Realizado por Dr. A. Martínez-Conde Dra P.
Mayor Dep. Bioquímica y Biología Molecular Fac.
Medicina Universidad Complutense de Madrid
6
Estructura del mismo canal de potasio en su forma
abierta mostrando el paso del ión
Texto K Selectivity Pore Close-up Only three
of the subunits are shown to provide a clearer
view of the pore. All seven of the possible K
locations are shown. However, only four, at most,
are occupied at the same time the other sites
contain waters. Residues 75-79 comprise the
selectivity pore and are shown as Sticks, colored
CPK. The mainchain CO's of these residues
coordinate the K ions. The water-filled cavity
is colored cyan. All of the waters shown here are
highly-ordered and thus differ from the "bulk"
water surrounding the channel. K at sites 2
4 Each K ion is coordinated by eight oxygens
with water between them. For example, K at site
4 is coordinated by the side chains and CO's of
Thr 75. K between sites The K ions are
coordinated by only four of the mainchain CO's.
For example, the K between sites 2 and 3 is
coordinated only by Val 76. A K is about to
enter the pore from the cavity. K at sites 1
3 The K ions are coordinated by eight mainchain
CO's. For example, K at site 1 is coordinated
by Gly 77 and Tyr 78.The pore is normally
occupied by two K ions and two waters. Concerted
movement of the two ions and their waters leads
to K exit from, or entry into the cell. The
scripted animation shows the sequence of steps in
the exit pathway. KEffluxMechanismThe steps
shown in the animation (See Morais-Cabral et al.,
Figs. 4 and 5.) State I Water-filled pore
The K that will soon exit is in the cavity.
State H K at site 4 The K enters the pore
and is replaced by another in the cavity. State
E K at site 3 The K moves up one position
(with the waters). Another K occupies the cavity
from the cytosol. State C K at sites 2 4
The K moves up another position. Intermediate
between site 2 and the extracellular site The K
is coordinated by only four of the mainchain
CO's prior to dissociation, first by Tyr 78 and
finally by Gly 79. Dissociation from
extracellular site The K binds briefly at site
0 and at the fully hydrated extracellular site,
then diffuses into the extracellular solution.
Script ends with K at sites 2 4. For multiple
cycles of ion efflux, the pathway resumes at
State C above. K entry follows the same pathway
in reverse direction. A single cycle takes about
10 nanoseconds to complete!
Realizado por Dr. A. Martínez-Conde Dra P.
Mayor Dep. Bioquímica y Biología Molecular Fac.
Medicina Universidad Complutense de Madrid
7
Realizado por Dr. A. Martínez-Conde Dra P.
Mayor Dep. Bioquímica y Biología Molecular Fac.
Medicina Universidad Complutense de Madrid
8
Realizado por Dr. A. Martínez-Conde Dra P.
Mayor Dep. Bioquímica y Biología Molecular Fac.
Medicina Universidad Complutense de Madrid
9
Canales Regulados por Ligandos
Canal de iones que se Abre por interacción con un
Ligando. Un ejemplo es el Canal de Na que es el
Receptor Nicotínico de Acetil-Colina. Se
encuentra en la membrana post-sináptica de nervio
o placa motora.
Cambio Conformacional
El Canal en Reposo se encuentra en su
conformación Cerrada,
Cuando se degrada la Acetil-Colina de la
hendidura sináptica, se producirá un descenso en
la concentración de este neurotransmisor. Esto
hace que se disocie del sitio de unión del Canal
de Na y como consecuencia se induzca un cambio
conformacional en el Canal que le hace pasar a la
forma Cerrada de Reposo
Un aumento en la concentración de Acetil-Colina
en la hendidura sináptica hace que este
neurotransmisor se una al sitio de unión del
Canal de Na y como consecuencia se induzca un
cambio conformacional en el Canal que le hace
pasar a la forma Abierta
Abierto
Cerrado
Cambio Conformacional
Realizado por Dr. A. Martínez-Conde Dra P.
Mayor Dep. Bioquímica y Biología Molecular Fac.
Medicina Universidad Complutense de Madrid
10
El Receptor Nicotínico de la Acetil-Colina
Structure of the Nicotinic Acetylcholine Receptor
PoreThe initial view of the AChR pore is from
the synaptic (or extracellular space). Each
subunit of the pentamer (a2bgd) is colored
individually. The two a subunits are red. The
four transmembrane helices are labeled at their
N-termini in the g subunit (cyan). The central
ion pore is formed by side chains from one side
of helix M2.The membrane thickness indicated is
the apolar region of the membrane. Side View
The protein is shown as Sticks from the plane of
the membrane. The M2 helices are blue the rest
are red. In the intact AChR, the N-termini of
each subunit connect to the ligand-binding domain
(at the top). The width and height of the pore
domain can be displayed (Å).
Pore Features Pore Surface The protein is shown
Spacefill. One subunit has been removed to allow
a view into the pore. The residues colored yellow
form a "hydrophobic girdle" that is likely to
have the gating function in AChR. The dark blue
ball represents a Na ion trapped at the level of
the maximal constriction. (The ion was inserted
into the 1OED.pdb coordinate file for
illustration.) Pore Gate The view is from the
top of the pore. The M2 helices are shown as
Backbone each is labeled at the N-termini by
subunit name. The hydrophobic girdle side chains
are shown as Sticks, colored yellow they are
labeled on the a subunit. Note that although
these residues are not identical in each subunit,
they are similar in polarity and size. The
"hypothetical Na ion" is shown as blue
Spacefill. The gate in this channel is
hypothesized to restrict passage of hydrated Na
ions. Compare the structure shown here with that
of say, the KcsA channel where main chain CO
groups interact with dehydrated K ions. The
energetic cost of removing water with apolar side
chains in this pore would be prohibitive.
Realizado por Dr. A. Martínez-Conde Dra P.
Mayor Dep. Bioquímica y Biología Molecular Fac.
Medicina Universidad Complutense de Madrid
11
Canal de iones que se Abre por interacción con
una Proteína Reguladora. Un ejemplo sería el
Canal de K de músculo cardiaco Regulado por las
Subunidades Gibg de la Proteína Gi. Está regulado
por el Receptor Muscarínico de de Acetil-Colina
ACETIL-COLINA
Transición Conformacional
RECEPTOR GPCR MUSCARÍNICO
K
Realizado por Dr. A. Martínez-Conde Dra P.
Mayor Dep. Bioquímica y Biología Molecular Fac.
Medicina Universidad Complutense de Madrid