Presentazione di PowerPoint - PowerPoint PPT Presentation
Title:
Presentazione di PowerPoint
Description:
Pertussis toxin (whooping cough disease) catalyzes ADP-ribosylation at a cysteine residue of Gia, making the inhibitory Ga incapable of exchanging GDP for GTP. – PowerPoint PPT presentation
Number of Views:77
Avg rating:3.0/5.0
Title: Presentazione di PowerPoint
1
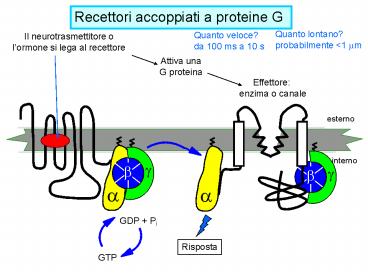
Recettori accoppiati a proteine G
2
(No Transcript)
3
(No Transcript)
4
The alpha subunit of the heterotrimeric G protein
is shown as a ribbon the guanine nucleotide is
spacefilled. P-alpha, P-beta, and P-gamma
indicate the three phosphoryl groups in the GTP
structure. As with most nucleoside triphosphates,
there is a magnesium ion associated with GTP. The
"ras-like" domain contains the catalytic residues
that promote GTP hydrolysis.
5
The nucleotide binding site in each GTP-binding
switch protein consists of loops that extend out
from a b-sheet, usually 6-stranded.
Three switch domains have been identified, that
change position when GTP substitutes for GDP on
Ga. These include residues adjacent to the
terminal phosphates of GTP and/or the Mg
associated with them.
6
The b subunit of the heterotrimeric G Protein has
a b-propeller structure, formed from
multiple repeats of a sequence called the
WD-repeat. The b-propeller provides a stable
structural support for residues that bind Ga.
7
The alpha subunit is usually modified by a fatty
acyl lipid anchor. The gamma subunit is usually
modified by an isoprenoid lipid anchor. Both
lipid anchors (zig-zag lines) permit lateral
diffusion, protein-lipid, and protein-protein
interactions.
8
(No Transcript)
9
(No Transcript)
10
(No Transcript)
11
(No Transcript)
12
Outline the cyclic-AMP and phosphoinositide
signal transduction cascades differentiate
between activation and inhibition of effector
proteins by G protein subunits.
13
(No Transcript)
14
- Turn off of the signal (when AC is activated)
- 1. Ga hydrolyzes GTP to GDP Pi. (GTPase).
- The presence of GDP on Ga causes it to rebind to
the inhibitory bg complex. - Adenylate Cyclase is no longer activated.
- 2. Phosphodiesterase catalyzes hydrolysis of
- cAMP ? AMP.
15
- Turn off of the signal (cont.)
- 3. Hormone receptor desensitization occurs. This
process varies with the hormone. - Some receptors are phosphorylated via
G-protein-coupled receptor kinases (GRK). - The phosphorylated receptor may then bind to a
protein arrestin that blocks receptor-G-protein
activation and promotes removal of the receptor
from the membrane by clathrin-mediated
endocytosis. - 4. Protein Phosphatase catalyzes removal by
hydrolysis of phosphates that were attached to
proteins via Protein Kinase A.
16
(No Transcript)
17
The Gs-alpha and Gi-alpha subunits both interact
with adenylyl cyclase isoforms. Their actions,
however, are opposite Gs stimulates and Gi
inhibits the synthesis of cyclic-AMP from ATP.
The actions of these two alpha subunits may be
differentiated in the laboratory by certain
bacterial toxins.
18
(No Transcript)
19
(No Transcript)
20
Cholera toxin catalyzes covalent modification of
Gsa. ADP-ribose is transferred from NAD to an
arginine residue at the GTPase active site of
Gsa. This ADP-ribosylation prevents Gsa from
hydrolyzing GTP. Thus Gsa becomes permanently
activated. Pertussis toxin (whooping cough
disease) catalyzes ADP-ribosylation at a cysteine
residue of Gia, making the inhibitory Ga
incapable of exchanging GDP for GTP. Thus the
inhibitory pathway is blocked. ADP-ribosylation
is a general mechanism by which activity of many
proteins is regulated, in eukaryotes (including
mammals) as well as in prokaryotes.
21
(No Transcript)
22
(No Transcript)
23
(No Transcript)
24
(No Transcript)
25
(No Transcript)
26
G proteins (guanine nucleotide (GTP) -binding
proteins) G proteins, once activated, will cause
the activation of several intracellulareffectors
adenyl cyclase, cGMP phosphodiesterase,
phospholipase C, phospholipase A2, and calcium or
potassium channels. l Gi proteins
(adenylate cyclase-inhibiting) - linked to
a2-adrenergic receptor m Gi1 protein m
Gi2 protein m Gi3 protein l Go
protein (Calcium or potassium channels
modulators) l Gq protein (Phospholipase C
activator) linked to a1-adrenergic
receptor l Gs proteins (adenylate
cyclase-stimulating) - linked to b-adrenergic
receptor
27
- Small GTP-binding proteins include (roles
indicated) - initiation elongation factors (protein
synthesis). - Ras (growth factor signal cascades).
- Rab (vesicle targeting and fusion).
- Ran (transport of proteins into out of the
nucleus). - Rho (regulation of actin cytoskeleton)
- All GTP-binding proteins differ in conformation
depending on whether GDP or GTP is present at
their nucleotide binding site. - Generally, GTP binding induces the active state.
28
Most GTP-binding proteins depend on helper
proteins GAPs, GTPase Activating Proteins,
promote GTP hydrolysis.
A GAP may provide an essential active site
residue, and/or promote a conformation that
favors catalysis. Ga of a heterotrimeric G
protein has innate capability for GTP hydrolysis.
However, RGS proteins, which are negative
regulators of G protein signaling, function as
GAPs to stimulate GTP hydrolysis by Ga.
29
GEFs, Guanine Nucleotide Exchange Factors,
promote GDP/GTP exchange. The activated receptor
(GPCR) serves as GEF for a heterotrimeric G
protein.
30
The regulation of G protein signalling
Left panel RGS proteins bind to G, stimulate GTP
hydrolysis, and thereby reverse G protein
activation. Right panel, the roles of a receptor,
G, and an RGS are completely analogous to the
GDSs, GDIs, and GAPs that regulate small
monomeric G proteins like Ras.
31
(No Transcript)
32
G protein Mutations Causing Disease
G protein alpha subunit in its GTP-bound form,
highlighting amino acids changed by point
mutations that cause human endocrine diseases.
Mutational replacements of red residues impair
GTP hydrolysis these sites are mutated in growth
hormone secreting tumors of the pituitary.
Replacement of either cyan residue produces an
inactive G protein alpha subunit, causing
pseudohypoparathyroidism. Bound nucleotide is
light green.