Presentazione di PowerPoint - PowerPoint PPT Presentation
1 / 57
Title:
Presentazione di PowerPoint
Description:
... (II) have no ZFS, small magnetic anisotropy and, in general, excited states far ... like high spin Mn(II) and high spin Fe(III), have small g anisotropy and first ... – PowerPoint PPT presentation
Number of Views:51
Avg rating:3.0/5.0
Title: Presentazione di PowerPoint
1
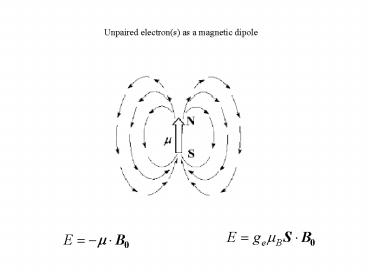
Unpaired electron(s) as a magnetic dipole
2
(No Transcript)
3
Dipole-dipole interaction
4
Nuclear relaxation due to the electron-nucleus
dipolar coupling Solomons equations
5
- Three times modulate the dipolar Hamiltonian
- Electron relaxation ts
- 2) Rotation tr
- 3) Chemical exchange tM
6
Each time contributes to the decay of the
correlation function
7
(No Transcript)
8
Contact relaxation
9
(No Transcript)
10
Contact shift
In the high field approximation
H.M. McConnell, D.B. Chesnut, J.Chem.Phys. 1958,
28, 107-117
Kurland and McGarvey approach indicates that ltSigt
may be orientation dependent due to spin-orbit
coupling
R.J. Kurland, B.R. McGarvey, J.Magn.Reson. 1970,
2, 286-301
11
Kramers doublets and magnetic parameters
? a 1,-1/2gt b 0,1/2gt c -1,-1/2gt
?- a -1,1/2gt b 0,-1/2gt c 1,1/2gt
a,b,c identify the three orbitals and depend on
the electron energy levels and on spin-orbit
coupling
Program available at www.cerm.unifi.it
Horrocks, Greenberg Biochim.Biophys. Acta (1973)
332, 38
12
g and Dc values in LS oxidized heme proteins
MetMbCN gx 0.93 gy 1.89 gz 3.45 ??ax
3.78?10-32 m3 ??rh -1.01?10-32 m3 Cytochrome
b5 gx 1.43 gy 2.23 gz 3.03 ??ax
2.83?10-32 m3 ??rh -1.06?10-32 m3 Cytochrome
c gx 1.25 gy 2.25 gz 3.06 ??ax
2.68?10-32 m3 ??rh -1.25?10-32 m3
13
Contact contributions in oxidized cytochrome b5
A0,1,2/h (in MHz) are the hyperfine coupling
constants for the ground and excited levels
where a1 is an heuristic parameter and the qi
angles are between the metal-ith-methyl direction
and the metal-pyrrole II axis
A2 a2
14
Average contact shift (in solution)
for A0/h A1/h A2/h 1 MHz
to be compared with the McConnell expression
The contact shift changes as a function of the
orientation of the molecule.
15
A magnetic field B0 orients the electron magnetic
moments cM (m3 mol-1) is the
magnetic susceptibility per mole ltmgt is the
average induced magnetic moment per particle
in lanthanides
16
Spin magnetic moment always orients along the
external magnetic field. Orbital contribution has
its own orientation within the molecule.
As a result, the projection of the total
electronic magnetic moment along B0 depends on
the orientation of the molecule, and is therefore
anisotropic.
17
?r rotational correlation time
18
Curie relaxation
19
NMR and electron relaxation times
- Relaxation and contrast agent
- Information on electron relaxation
- Signals observed far from metal
- If close to metal, only in high
molar excess and under fast
chemical exchange
Line broadening 300 Hz for a proton at 5.5 A
from the metal
- High resolution NMR
- Shift reagents
- Local structural information
- Solution structure determination
20
NMR and electron relaxation times
- Relaxation and contrast agent
- Information on electron relaxation
- Signals observed far from metal
- If close to metal, only in high
molar excess and under fast
chemical exchange
Line broadening Dn J for a proton at 9 A from
the metal
for a proton at 4 A from the metal
- High resolution NMR
- Shift reagents
- Local structural information
- Solution structure determination
21
Curiedipolar relaxation (S 5/2 ts 2
10-10 s tr 10-8 s 500 MHz )
22
(No Transcript)
23
(No Transcript)
24
(No Transcript)
25
Electron relaxation
Direct Raman
Orbach
26
Additional terms in the static Hamiltonian
I?A?S hyperfine coupling with the metal
nucleus S?D?S zero field splitting
S?g?B0 g-anisotropy important when
Fluctuations cause electron relaxation
27
Electron relaxation due to modulation of ZFS
transient ZFS
correlation time for electron relaxation
28
S 1/2 ions like Cu(II) have no ZFS, small
magnetic anisotropy and, in general, excited
states far above the ground state in energy.
Electronic relaxation times are therefore long.
Cu(II) aqua ion
Best fit r 0.27 nm ?c 2.6?10-11 s (?s
3?10-10 s )
29
Cu(II) in glycerol
Nuclear relaxation is modulated by electron
relaxation time. Profiles different from SBM due
to hyperfine coupling with the metal nucleus.
288 K
264 K
298 K
278 K
312 K
water, 298 K
30
1H NMR spectra of oxidized spinach plastocyanin
at 800 MHz at 298 K
31
S 5/2 ions, like high spin Mn(II) and high spin
Fe(III), have small g anisotropy and first
excited levels much higher than the ground level,
since they arise from a different free ion term.
In these cases, modulation of the quadratic ZFS
is probably the most efficient relaxation
mechanism in solution
Mn(II) aqua ion
Best fit r 0.27 nm ?r 3?10-11 s ?s0
3.5?10-9 s ?v 5.3?10-12 s A/h 0.8 MHz
R2
R1
32
Mn(II) concanavalin
D 0.04 cm-1
298 K
278 K
33
Fe(III) aqua ion
Best fit at 298 K r 0.26 nm ?r 5.3?10-11 s
?t 0.095 cm-1 ?v 5.3?10-12 s ?M 3.8?10-7
s A/h 0.43 MHz
(?) 278 K, (?) 288 K, (?) 298 K, (?) 308 K
R2 (?) 308 K
34
Fe(III) in glycerol
in 60 glycerol
in water
35
HS Fe(III)
contact
contact
contact
contact
Dip.
Dip.
Dip.
Curie
Curie
Curie
Curie
Curie
r 5 Å
36
(No Transcript)
37
- Oxidized rubredoxin
- ?C2H2 signals of 2H labeled cysteines
- hyperfine coupling constants ? 1-3 MHz for
protons. - Two 2H? protons appear
- downfield (180 and 150 ppm)
- the other two appear
- upfield (-10 ppm, overlapped)
J.L. Markley at al., J.Am.Chem.Soc., 1995
38
Met-myoglobin
39
Electronic configuration of LS FeIII Heme
X
Fe(III)
X
dxz, dyz
dxy
X cylindric ligand
D4h
S 1/2 ions like low spin iron(III), with ground
levels deriving from an orbitally degenerate
ground level in cubic symmetry, may havelow-lying
excited states therefore, Orbach processes are
likely to be very efficient and short relaxation
times are expected .
40
Electronic configuration of LS FeIII Heme
N
C
F
e
dxz
N
dyz
dxy
N
C2
first
excited state
41
Met80Ala cyano-cytochrome c
8-CH3
1-CH3
3-CH3
5-CH3
Banci, Bertini at al. JBIC (1996)
42
Cytochrome b5
8-CH3
5-CH3
1-CH3
3-CH3
Banci, Bertini, et al. Inorg.Chem. (1994)
Arnesano, Banci, Bertini, Felli Biochemistry
(1998)
43
Co(II)
glycerol, 264 K
water, 298 K
Best fit at 298 K in water r 0.27 nm ?s
5?10-12 s A/h 0.4 MHz
glycerol, 298 K
44
Co(II)-azurin
45
Ni(II) aqua ion, Ni(II) in glycerol, Ni(II)
carbonic anhydrase
Best fit in water r 0.27 nm ?s0 5?10-12 s ?v
2.2?10-12 s A/h 0.2 MHz
water
in glycerol at 264 (?) and 298 (?) K Ni(II)
bovine carbonic anhydrase II at pH 6.0 and 298 K
(?)
46
300 MHz 1H NMR spectrum of nickel(II)-substituted
azurin at pH 7.0 and 303 K
47
Ln(III) aqua ions
(?) Sm, (?) Pr, (?) Yb, (?) Er, (?) Ho, (?) Dy
48
Ln(III) electron relaxation
- ?s values obtained from
- the fit of the NMRD profiles (?),
- the fit with inclusion of ZFS effects (?)
- estimated from 1H relaxation measurements (?)
49
Predicted Curie line broadening at a field of
11.7 T (500 MHz) for a proton at 5 Å from the
lanthanide ion in a protein with tR of 4 ns (MW ?
104 Da), without (?) and with (?) inclusion of
the contribution from the first excited J
manifold.
50
Predicted Curie line broadening as a function of
the distance of the proton from the lanthanide
ion
detection threshold for a cross peak in a HSQC
(1H-15N or 1H-13C) experiment
detection threshold for the paramagnetic line
broadening in a protein of MW ? 104 Da
51
Predicted pseudocontact shifts
52
Thickness of the distance shells where meaningful
PCS can be measured and average number of protons
in each shell (in parenthesis)
53
Calculated ratio between PCS and Curie line
broadening, providing an estimate of the
resolution of pseudocontact shifted peaks in a 1D
experiment
54
resolution vs. PCS for the lanthanide series
55
Gd(III) aqua ion, in glycerol
Best fit in water r 0.31 nm ?r 4?10-11 s
?s0 1.3?10-10 s ?v 16?10-12 s
glycerol, 298 K
glycerol, 312 K
water, 298 K
56
(No Transcript)
57
Bulk susceptibility shift (Evans method for
measuring solute magnetic susceptibility)
The shifts of a probe substance differ in the two
solutions, A and B, and two different signals are
observed. The difference in chemical shift
separation in presence and absence of solute in
A, if the concentrations of the paramagnetic and
diamagnetic solutes are the same, is directly
related to c