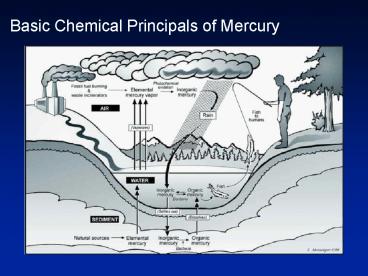
Basic Chemical Principals of Mercury - PowerPoint PPT Presentation
1 / 30
Title:
Basic Chemical Principals of Mercury
Description:
Water. Air. Natural concentrations: 5 to 100 pM (1 20 ng /L) Hgo (l) dissolution ... Concentration vs Activity. HgS = Hg2 S2- log Ksp = -53. Ksp = (Hg2 ) (S2 ... – PowerPoint PPT presentation
Number of Views:45
Avg rating:3.0/5.0
Title: Basic Chemical Principals of Mercury
1
Basic Chemical Principals of Mercury
2
Solid-Water Interface
Mineralogical transformation
biomineralization
precipitation
dissolution
Mn
Reduction
Oxidation
Mnx
release
Bacteria
deposition
Organic Matter
Mineral
adsorption
Organic ligand
desorption
Soil Profile
complexation
Aqueous Metal Ion
degradation
Metal-Organic Complex
Surface complex
3
(No Transcript)
4
Hgo (g)
Hg(II) (s)
Air
deposition
volatilization
Water
reduction
Hg(II)
Hgo (aq)
Hgo (l)
oxidation
dissolution
Natural concentrations 5 to 100 pM (1
20 ng /L)
ng/L ppt µg/L ppb mg/L ppm
5
Hgo (g)
Hg(II) (s)
Air
deposition
volatilization
Water
reduction
Hg(II)
Hgo (aq)
Hgo (l)
oxidation
dissolution
Hgo (l) ?? Hgo (aq) K 10-7.3 (mol/L) Hgo
(g) ?? Hg (aq) K 2.56 x 10-3 (mol / L
atm)
6
Morel et al., 2002
7
Hgo (g)
Hg(II) (s)
Air
Water
reduction
Hg(II)
Hgo (aq)
Hgo (l)
oxidation
dissolution
8
Oxidation-Reduction Reactions
reduction
Hg(II)
Hgo (aq)
- Microbially mediated (dominant)
- Photoreduction
oxidation
Hgo (aq)
Hg(II)
- Limited in freshwater
- Particle surfaces catalyze O2 oxidation of
Hg-halides (HgCl2, for example)
9
Hgo (g)
Hg(II) (s)
Air
Water
reduction
Hg(II)
Hgo (aq)
oxidation
Hg2, HgCl2o, Hg(OH)2o, Hg(SH)2o, HgS(SH)-,
CH3Hg(SH)o
Natural concentrations 5 to 100 pM (1
20 ng /L)
10
Ion Coordination Hg(II)
Hg2 nH2O
11
Solutions
Speciation Differences in molecular configuration
Hg2, HgCl2o, Hg(OH)2o, Hg(SH)2o, HgS(SH)-,
CH3Hg(SH)o
12
Ion Complexes
Cation Anion (termed ligand) gt
Complex Hg2 Cl- ? HgCl- Keq 107.2
(association reaction) Hg(SH)2o ? Hg2
2 HS- log K -36.6 (dissociation
reaction) Kdiss (Hg2) (HS-)2 / (Hg(SH)2o)
DG -RT ln K
13
Oxic (Aerated) Waters
Morel et al. (2002)
14
Sulfide and Methyl Mercury
SO42-
HgS(HS)- Hg(HS)2 Hg(Sn)HS-
reduction
MeHg
SRB
Hgo (aq)
Hg(II)
oxidation
H2S, HS-
15
Guadalupe River Watershed
16
San Francisco Bay, Stinky Mud
17
Energy Yield
Electron Donor (food)
Electron Acceptor (breathing)
CH2O ? CO2
DGdonor - DGacceptor
SO42- ? H2S
NH4 ? NO3-
Fe3 ? Fe2
Fe2 ? Fe3
NO3- ? NH4
H2S ? SO42-
O2 ? H2O
Energy
Energy
18
Sulfide Complexes of Hg
Hg(SH)2o HgS(SH)- Hg(Sn)SH-
Hg2 HS-
19
Methyl Mercury (MeHg)
SRB
Hg(HS)2 HgS(HS)-
MeHg
MeHg CH3HgS- CH3HgCl CH3HgOH
20
Methylated Species of Hg
21
Interaction with Solids
Hgo (g)
Hg(II) (s)
Air
deposition
volatilization
Water
reduction
Hg(II)
Hgo (aq)
Hgo (l)
oxidation
Dissolution/precipitation
HgS
Sediment
22
Mineral Solubility
HgS Hg2 S2- log Ksp -53
What about other Hg(II) species?
23
Role of Sulfide
with So
24
Interaction with Solids
25
Ion Retention
adsorption
desorption
Aqueous Metal Ion
26
Adsorption Chemical versus Electrostatic
(strong) (weak)
- -
Hg2
27
Cylcing of Mercury
28
(No Transcript)
29
Mineral Solubility
HgS Hg2 S2- log Ksp -53
Ksp (Hg2) (S2-)
Concentration vs Activity
30
Calculating Activity Coefficients
B is a temperature dependent constant (0.33 _at_ 25
C) a is an effective ion size parameter