Cell Voltages - PowerPoint PPT Presentation
Title:
Cell Voltages
Description:
Title: Matter and Measurement Author: B. Venkataraman Last modified by: B. Venkataraman Created Date: 8/21/2000 5:10:42 PM Document presentation format – PowerPoint PPT presentation
Number of Views:113
Avg rating:3.0/5.0
Title: Cell Voltages
1
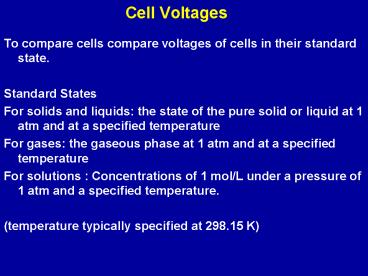
Cell Voltages
- To compare cells compare voltages of cells in
their standard state. - Standard States
- For solids and liquids the state of the pure
solid or liquid at 1 atm and at a specified
temperature - For gases the gaseous phase at 1 atm and at a
specified temperature - For solutions Concentrations of 1 mol/L under a
pressure of 1 atm and a specified temperature. - (temperature typically specified at 298.15 K)
2
- DGro
DEo
n F
- DEo is the standard cell voltage or potential of
a standard cell - when all reactants and products
are in their standard state. - DGro - n F DEo
DEo gt 0 spontaneous cell reaction under
standard conditions
3
A standard Cr3(aq)Cr(s) and a standard
Co2(aq)Co(s) half cell are connected to make a
galvanic cell. The voltage of the cell equals
0.464 V at 25oC. Write an equation to represent
the reaction taking place in the cell and
calculate its DGor. Cr(s) ? Cr3(aq)
3e- anode Co2(aq) 2e- ? Co(s) cathode Overal
l cell reaction 2Cr(s) 3 Co2(aq) ? 2Cr3(aq)
3 Co(s) DEo 0.464 V DGor - n F DEo -
(6 moles) (9.64853 x 104 coulomb/mole) (0.464
V) - 2.69 x 105 J or - 269 kJ
4
Standard Reduction Potentials
- Under standard conditions
- DEo Eo (right half cell) - Eo (left half
cell) - For a galvanic cell
- DEo Eo (cathode) - Eo (anode)
- where the Eo are the standard reduction
potentials of the electrodes. - For DEo gt 0 spontaneous cell reaction
- To determine which of two half cells will be the
anode and which the cathode compare standard
reduction potentials
5
Cr3(aq) 3e- ? Cr(s) Eo(Cr3Cr) - 0.744
V Co2(aq) 2e- ? Co(s) Eo(Co2Co) - 0.28
V Eo(Co2Co) gt Eo(Cr3Cr)
6
- Define the following half reaction to be the
reference - 2 H(aq, 1 M) 2e- -gt H2 (g, P 1 atm) Eo
0 V - All standard reduction potentials are determined
relative to this reference. - If the standard reduction potential of a half
reaction is gt 0 gt greater tendency to be reduced
relative to H(aq, 1 M) - If standard reduction potential of a half
reaction lt 0 gt lower tendency to be reduced
relative to H(aq, 1 M)
7
In general, the more positive the standard
reduction potential, the greater the
electron-pulling power of the reduction half
reaction, and therefore the more oxidizing the
species The more negative the standard reduction
potential, the greater the electron-donating
power of the oxidation half reaction, and
therefore the more reducing the species
8
(No Transcript)
9
Variation of standard reduction potentials. The
most negative values occur in the s block and the
most positive values occur close to fluorine.
10
- The standard potential of an electrode can be
determined by setting up a standard cell in which
one electrode has a known standard potential and
measuring the resulting cell voltage. - For example, the standard potential of a zinc
electrode is - -0.76 V, and the standard emf of the cell
- Zn(s) Zn2 (aq) Sn4 (aq), Sn2 (aq) Pt
(s) is 0.91 V - DEo Eo (cathode) - Eo (anode)
- 0.91 V Eo (Sn4 (aq), Sn2 (aq) ) - Eo
(Zn(s) Zn2 (aq) ) - Eo (Sn4 (aq), Sn2 (aq) ) 0.91 V Eo
(Zn(s) Zn2 (aq) ) - 0.91V - 0.76 V 0.15 V
11
Using standard reduction potentials Strong
oxidizing agents - have large positive standard
reduction potentials Examples F2, MnO4-, H2O2 O2
(in acidic medium) is a fairly strong oxidizing
agent. Strong reducing agents - have large
negative standard reduction potentials Examples
Na, Li
12
Electrochemical series list of relative
strengths of oxidizing and reducing agents. The
strongest oxidizing agents are at the top of the
table the strongest reducing agents are at the
bottom
M(s) 2H(aq) ? M2(aq) H2(g) spontaneous if
Eo(M2M) lt 0
13
Disproportionation a single species is both
reduced and oxidized. Must be able to both give
up and accept electrons Half reaction in which
the species is reduced must have a larger
reduction potential than the half reaction in
which it is oxidized. Is Fe2(aq) in its
standard state unstable with respect to
disproportionation at 25oC? Fe3(aq) e- ?
Fe2(aq) Eo 0.771 V Fe2(aq) 2e- ? Fe(s) Eo
-0.477 V Overall disproportionation reaction 3
Fe2(aq) ? 2 Fe3(aq) Fe(s) DEo -0.477 -
0.771 -1.218 V No disproportionation
14
Effect of Concentration on DE
- DGr DGro RT ln Q
- DGr - n F DE
- DGro - n F DEo
- - n F DE - n F DEo RT ln Q
- DE DEo - (RT/ n F ) ln Q Nernst Equation
- relates cell voltage with concentrations of
reactants and products (through Q)
15
- At 25.00oC (298.15 K), R T / F 0.025693 V
- DE DEo - (0.025693 / n ) ln Q
- The reduction potential of a non-standard half
cell is - E Eo - (RT/ nhc F ) ln Qhc
- For Zn2 (aq) 2 e- -gt Zn(s)
- E Eo - (RT/ 2 F ) ln (1 / Zn2(aq)
16
Ion-Selective Electrodes
- pH or concentration of ions can be measured by
using an electrode that responds selectively to
only one species of ion. - In a pH meter, one electrode is sensitive to the
H3O(aq) concentration, and the other electrode
serves as a reference. - A calomel electrode has a reduction half
reaction - Hg2Cl2 (s) 2 e- -gt 2 Hg(l) 2 Cl- (aq) Eo
0.27 V - When combined with the H(aq)/H2(g) electrode,
the overall cell reaction is - Hg2Cl2 (s) H2 (g) -gt 2 H (aq) 2 Hg(l) 2
Cl- (aq)
17
- Q H(aq)2 Cl- (aq)2 / PH2
- If PH2 is held at 1 atm then Q H(aq)2 Cl-
(aq)2 - DE DEo - (RT/ n F ) ln H(aq)2 Cl- (aq)2
- The Cl- (aq) is held constant since the calomel
electrode consists of a saturated solution of
KCl. - DE depends only on H(aq).
- Other electrodes are selectively sensitive to
ions such as Ca2, NH4, Na, S2-.