The Chemistry of Life - PowerPoint PPT Presentation
1 / 21
Title:
The Chemistry of Life
Description:
In non-scientific terms = STUFF like books, submarines, belly button lint. ... (except memories etc) So if everything is matter, how do we divide matter up? ... – PowerPoint PPT presentation
Number of Views:36
Avg rating:3.0/5.0
Title: The Chemistry of Life
1
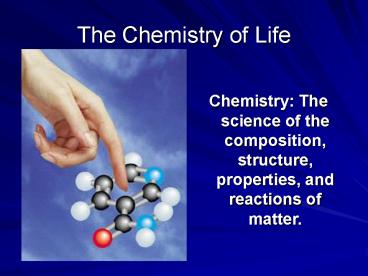
The Chemistry of Life
Chemistry The science of the composition,
structure, properties, and reactions of matter.
2
Whats this matter?
- Matter anything that occupies space (has volume)
and has mass - In non-scientific terms STUFF like books,
submarines, belly button lint - Everything is matter (except memories etc)
- So if everything is matter, how do we divide
matter up?
3
(No Transcript)
4
Remember, the smallest division of an element
that retains the characteristics of the element
is
- An atom
- Elements are made of a single kind of atom and
cannot be broken down by chemical means into
simpler substances. - Most common elements in living things C,H,O,N,S,P
5
(No Transcript)
6
So EVERYTHING IS MATTER AND CONTAINS ATOMS, BUT
WHAT ARE ATOMS MADE OF???????
- Atoms are composed of protons, neutrons, and
electrons.
7
The Atom
- Protons and neutrons in the nucleus
- Electrons move about the nucleus in orbitals.
- Pe in neutral
- atom
- Subatomic particles
- Protons - positive charge
- Electrons - negative charge
- Neutrons - no charge
8
(No Transcript)
9
Electrons
- Located outside of the nucleus
- Unless otherwise indicated the number of
electrons (-) is the same as the number of
protons () in a neutral atom (0). - Electrons orbit the nucleus in levels.
- Only electrons in the outermost energy level are
involved in chemical reactions
Remember Number of electrons number of
protons in neutral atom
10
Outer Shell Electrons
- Atoms seek to have a full outer shell of
electrons (8). - So, if an atom has five, it will seek out a way
to share or steal three electrons with other
atom(s). - Atoms with full shells are very stable
- Example Group 8ANoble Gas are unreactive
11
Electrons are found on energy levels
- The first energy level is closest to the nucleus
and fills with 2 electrons. - The second energy level is a bit further from the
nucleus and fills with 8 electrons - The third energy level is even further away from
the nucleus and fills with 8 electrons.
12
Electron Arrangements
13
Atomic Number
- The number of protons
- All atoms of same element have same atomic number
- Atomic number gives identity to element
Periodic table is arranged by order of atomic
number!
14
- Isotopes
- Atoms of the same element that have a different
number of neutrons are called isotopes. - Isotopes are atoms of the same element (same
protons) but different mass numbers
15
Mass Number
- Mass number is the Number of protons Number of
neutrons. - It is not a whole number because it takes into
account the relative amounts of isotopes.
16
How many protons and electrons in each isotope?
Atomic ?
17
How about neutrons and mass number?
18
Elements are Organized in the Periodic Table
- Back cover of text
- Elements symbolized by 1, 2, or 3 letters
- What element is
- C -Cl
- O -F
- Shows
- Atomic Number
- Chemical Symbol
- Atomic Mass (Not whole-accounts for isotopes)
- Mass Number (whole number of protons plus
neutrons)
19
Lets Practice
- Element?
- Atomic Number?
- Atomic Mass?
- How many protons?
- How many neutrons?
- How many electrons in a neutral atom?
20
Lets Practice
- Element?
- Atomic Number?
- Atomic Mass?
- How many protons?
- How many neutrons?
- How many electrons in a neutral atom?
21
End of Notes
Hooray!!!!
- Homework
- Atomic Structure Worksheet
- Read pages 28-31
- Complete the vocab for section 2.1 and parts of
2.2 (ask teacher)