DIAZINES - PowerPoint PPT Presentation
Title:
DIAZINES
Description:
DIAZINES. Diazines -Reacts even less readily with electrophiles than pyridine -Reacts easily with nucleophiles ... Structure - Tautomerism. React. with E-files ... – PowerPoint PPT presentation
Number of Views:1036
Avg rating:3.0/5.0
Title: DIAZINES
1
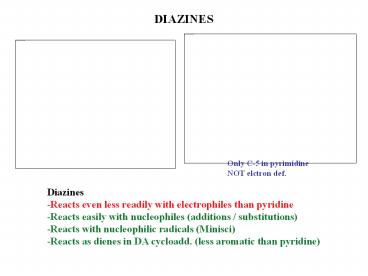
DIAZINES
Only C-5 in pyrimidine NOT elctron def.
Diazines -Reacts even less readily with
electrophiles than pyridine -Reacts easily with
nucleophiles (additions / substitutions) -Reacts
with nucleophilic radicals (Minisci) -Reacts as
dienes in DA cycloadd. (less aromatic than
pyridine)
2
Reactions with electrophiles
-Protonation -N-alkylation -Ox. to N-oxides
(H2O2, peracids) -Pract. no E-fil Ar subst. (C-5
pyrimidine) -Halogenation by add. / elim.
mechanisms
c.f. pyridines
weaker bases than pyridine
3
Reactions with nucleophiles
Addition - Rearomatization
RMet RLi, RMgX ox DDQ, KMnO4
1,2- add 1,4-add
Add. av NH2- (Chichibabin)
4
Substitution on halodiazines
Nucleophiles -ammonia / amines -thiolates -malona
tes etc. -water / alchohols / alchoxides
Leaving groups -halogen -OMe -SO2Me
rel. soft
5
With hard Nu
Pd-cat. couplings
6
Metallation
7
Radical reactions (Minisci)
Cycloadditions (DA)
8
Oxydiazines
Structure - Tautomerism
React. with E-files
More electron rich, reacts easier with E-files
than diazines OH o/p directing
9
React. with Nu-files
Nu Ar subst
More complex mech.
10
Undheim - Metaphase Inhibitors
Metaphase
Prophase
Anaphase
Telophase
11
N-Deprotonation / Alkylation
O-silylation / N-alkylation
12
N-Deprotonation / Alkylation
N-alkylation / C-alkylation
N-alkylation / O-alkylation
13
C-Deprotonation / Metallation
Excess base Because of NH
14
Replacement of oxygen
Halogenation
Oxo ? thio
15
Cycloadditions
Cancer
Psoralenes - Psoriasis
16
Aminodiazines
- Exists as aminodiazines (not imino)
- -NR2 electron donor Stronger bases
- -NR2 electron donor Participates easier in E-fil
Ar subst. - Diazotation reactions
- Dimroth rearrangement
Dimroth
17
Synthesis of Pyridazines
Carbonyl condensations
Cycloadditions
18
Synthesis of Pyrimidines
Carbonyl condensations etc.
Cycloadditions
19
Bioactive Pyrimidines
DNA bases
Base pairs
Double a-helix
20
Anticancer comp.
Antivirals
HIV (RT -inhibitors)
Barbiturates (old sedatives)
21
Synthesis of Pyrazines
Carbonyl condensations etc.
Bioactive Pyrazines/Pyridazines Pteridines
Bacteria synthezize folic acid