Aufbau Principle PowerPoint PPT Presentations
All Time
Recommended
Aufbau Principle
| PowerPoint PPT presentation | free to download
Aufbau Principle e- fill lowest energy levels 1st. Half-filled and filled states are preferred. 1s 2s 2p 3s 3p 3d 4s 4p 4d 4f 5s 5p 5d 5f 5g 6s 6p 6d 6f 6g 6h
| PowerPoint PPT presentation | free to view
Ratner Ch. 9.5-, Engel Ch. 10.5-, Pilar Ch. 10 Modern Quantum Chemistry, Ostlund & Szabo (1982) Ch. 3.3 ... (1 nucleus + 2 electrons) (Review) ...
| PowerPoint PPT presentation | free to view
Zn = [Ar]4s23d10. Not all transition elements follow simple order; the reasons are complex. ... Exceptions to the trend is a function of electron electron interactions ...
| PowerPoint PPT presentation | free to view
Section 5.3 Electron Configuration Apply the Pauli exclusion principle, the aufbau principle, and Hund's rule to write electron configurations using orbital diagrams ...
| PowerPoint PPT presentation | free to view
Pre AP Physical Science Bond Types Basic Principle: electrons occupy lowest energy levels available Aufbau Principle Diagonal Rule 1 1 s value of energy ...
| PowerPoint PPT presentation | free to download
Electron Configurations Electron Configuration Electron configuration the _____ of electrons in an atom Aufbau Principle Each electron must occupy the ...
| PowerPoint PPT presentation | free to download
The Periodic Table. Periodic Table. Dmitri Mendeleev (1834-1907) ... Modern Periodic Table. s- and p-orbitals. Aufbau' Principle: filling orbitals. 1s. 2s ...
| PowerPoint PPT presentation | free to download
84.443/543 Advanced Inorganic Chemistry The f orbitals The Aufbau Principle The loss of degeneracy in multi-electron atoms or ions results in electron configurations ...
| PowerPoint PPT presentation | free to download
Electron Arrangement and the Periodic Table Valence Electrons Energy Levels and Sublevels Electron Configuration and the Aufbau Principle Abbreviated Electron ...
| PowerPoint PPT presentation | free to download
Electron Configurations Of Atoms & Ions Men & their Rules Aufbau Principle: Start at the beginning (Electrons enter lowest energy level 1st) Pauli Exclusion Principle ...
| PowerPoint PPT presentation | free to download
Electron Configurations Of Atoms & Ions Men & their Rules Aufbau Principle: Start at the beginning (Electrons enter lowest energy level 1st) Pauli Exclusion Principle ...
| PowerPoint PPT presentation | free to download
The Aufbau ('building up') principle: lowest energy orbitals are ... distance from the nucleus outweighs effective nuclear charge for atomic radii down a group. ...
| PowerPoint PPT presentation | free to download
All truths are held to be derivable from the world-description plus definitional ... All charge' truths derivable from complete enough truth in the O-vocabulary. ...
| PowerPoint PPT presentation | free to download
Chapter 8 Electron Configuration and Periodicity Overview Electron Structure of Atoms Electron spin and the Pauli Exclusion Principle. Aufbau Principle and the ...
| PowerPoint PPT presentation | free to download
Electron Configurations Electron Configuration _____ the arrangement of electrons in an atom Aufbau Principle Each electron must occupy the _____energy level ...
| PowerPoint PPT presentation | free to download
Chapter 4 Electron Configurations Pauli Exclusion Principle No two electrons in an atom can have the same four quantum numbers. Aufbau principle An electron occupies ...
| PowerPoint PPT presentation | free to download
number of columns depend on max number of electrons. exceptions to Aufbau principle ... Zeff increase outweighs shielding for given l. deviations from trends ' ...
| PowerPoint PPT presentation | free to download
Elements of Plan of the DMV (1993-95) for a. Distributed ... DC-1, March 1995, Dublin, Ohio. OCLC/NCSA Metadata Workshop. DC-5, October 1997, Helsinki ...
| PowerPoint PPT presentation | free to view
Biometry and Epidemiology, Data Modeling and Information Systems in Medicine, ... Computer Simulation and Biometry. Information Management in Health Care ...
| PowerPoint PPT presentation | free to view
Modern Theory of the Atom: Quantum Mechanical Model Frequencies in Chemistry Electron Configuration & PT Principle Energy Levels Sublevels Orbitals hold 2 electrons ...
| PowerPoint PPT presentation | free to download
The Quantum Mechanical Model of the Atom Electrons act as waves Heisenberg Uncertainty Principle Schr dinger's Wave Equation Quantum Theory: describes mathematically ...
| PowerPoint PPT presentation | free to view
Chemistry I Electrons in Atoms Chapter 5 46.) What is the Pauli Exclusion Principle? A maximum of two electrons can occupy an orbital and they must have opposite spins.
| PowerPoint PPT presentation | free to view
Where are electrons found? Heisenberg uncertainty principle: It is impossible to know both the momentum and the position of a particle with certainty.
| PowerPoint PPT presentation | free to view
The way electrons are arranged around the nucleus. ... Aufbau Diagram for Beryllium. Aufbau Diagram for Boron. Aufbau Diagram for Carbon ...
| PowerPoint PPT presentation | free to view
Bohr Model (cont.) Energy Level Postulate. electrons can have only ... Bohr Equation ... exclusion principle, which of the following orbital diagrams are possible? ...
| PowerPoint PPT presentation | free to view
'n' is a principal energy level that can be divided into sublevels ... the lowest principle energy level, sublevel, and orbitals FIRST because these ...
| PowerPoint PPT presentation | free to view
Duality of light explained photoelectric effect! ... Electrons have a dual wave-particle nature. Heisenberg Uncertainty Principle ...
| PowerPoint PPT presentation | free to view
Spin and the Pauli Exclusion Principle. No two electrons in an atom can ... mixture of properties, often look metallic but without conductivity or ductility ...
| PowerPoint PPT presentation | free to view
Pauli exclusion principle ... O2-plasma Ar plasma Nature Artificial * How we using plasma in semiconductor fabrication? Etching Deposition thin film * e ...
| PowerPoint PPT presentation | free to view
New Energy Level Diagrm for Multi-electron Atoms. Pauli Exclusion Principle: ... Easier way: Use the periodic table. Exceptions. Electron Configurations of Ions ...
| PowerPoint PPT presentation | free to view
Bohr's Model. Explains emission spectrum of H ... Can also use orbital box or line diagrams. Let's take a look. 15. Pauli Exclusion Principle ...
| PowerPoint PPT presentation | free to view
Equantum = hv. E is energy. H is Planck's constant ... Principle Quantum Number (n)-indicates the size of the electron cloud and is ...
| PowerPoint PPT presentation | free to view
Every electron on an atom is in a definite state (energy level) with a ... Rules for determining the ground state of an atom. The Pauli exclusion principle. ...
| PowerPoint PPT presentation | free to view
The first 'p' sublevel ... All orbitals within the highest sublevel are completely filled ... electrons in the same sublevel, but different principle energy ...
| PowerPoint PPT presentation | free to view
Title: The Periodic Table Author: eric potma Last modified by: eric potma Created Date: 7/9/2006 9:44:16 PM Document presentation format: On-screen Show
| PowerPoint PPT presentation | free to download
Quantum Mechanical Model: Electron Configurations Chemistry 11 Electron Configurations It is the nature of things to seek the lowest possible energy.
| PowerPoint PPT presentation | free to view
Electron Configuration Mapping the electrons Electron Configuration The way electrons are arranged around the nucleus. Quantum Mechanical Model 1920 s Werner ...
| PowerPoint PPT presentation | free to download
Homework 23) Draw electron dot structures for atoms of the following elements: Homework 23) Draw electron dot structures for atoms of the following elements: a ...
| PowerPoint PPT presentation | free to download
Chapter 4 Homework Due Monday, September 22nd P 118 120 #6, 23, 30, 31, 32, 33, 40, 43, 46, 47
| PowerPoint PPT presentation | free to view
Atomic Structure Electron Configuration Scandium 3-D video (2:31) 3-D Graphic Examples of Atomic Orbitals ...
| PowerPoint PPT presentation | free to view
Thursday, Oct. 31st: A Day (1 :05 release) Friday, Nov. 1st: B Day Agenda Homework Questions/Collect Finish Section 3.3: Electron Configuration ...
| PowerPoint PPT presentation | free to view
Unit 3: Atomic Structure
| PowerPoint PPT presentation | free to download
Electronic structure in atoms AH Chemistry, Unit 1(a) Bohr s theory cannot explain spectra for atoms more complex than hydrogen, which show sub-lines.
| PowerPoint PPT presentation | free to download
Electron Configurations Today we are going to assign addresses to each electron. The energy diagram shows where we put the electrons in every atom
| PowerPoint PPT presentation | free to download
chemistry Chapter 5
| PowerPoint PPT presentation | free to download
Electron Configurations 1926 further refined Bohr s model of the atom by developing the quantum mechanical model New Model of the Atom Comparison to Bohr s ...
| PowerPoint PPT presentation | free to view
What is the difference between ground and excited state electrons? 6. How is light produced? 7. Why is light of different color? 8. What color is high frequency light?
| PowerPoint PPT presentation | free to view
Quantum Theory and the Electronic Structure of Atoms Part 2 Unit 4, Presentation 1
| PowerPoint PPT presentation | free to view
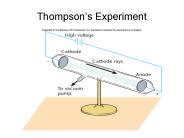
Thompson s Experiment Rutherford s Experiment: Explanation The Wave Nature of Light Light as electromagnetic waves: polarization, interference, diffraction ...
| PowerPoint PPT presentation | free to download
Electron Configuration Peg Ellis INTRODUCTION Modern Atomic View: The world of the atom is made up of waves and probability, The speed and location are subject to ...
| PowerPoint PPT presentation | free to download
CHEMISTRY Matter and Change Chapter 5: Electrons in Atoms
| PowerPoint PPT presentation | free to download
Ch. 5 How are an atom s electrons configured?
| PowerPoint PPT presentation | free to view
What s coming up??? Oct 25 The atmosphere, part 1 Ch. 8 Oct 27 Midterm No lecture Oct 29 The atmosphere, part 2 Ch. 8 Nov 1 Light, blackbodies, Bohr Ch. 9
| PowerPoint PPT presentation | free to download
Chapter 13: Electrons in the Atom College Prep Chemistry Orbital Interactive The lowest rung of the ladder corresponds to the lowest energy level.
| PowerPoint PPT presentation | free to download
Atomic Structure From Indivisible to Quantum Mechanical Model of the Atom V.Montgomery & R.Smith * s, p, and d describe the shape of the orbital.
| PowerPoint PPT presentation | free to view