I' The Aufbau Principle and the Periodic Table - PowerPoint PPT Presentation
1 / 11
Title:
I' The Aufbau Principle and the Periodic Table
Description:
Zn = [Ar]4s23d10. Not all transition elements follow simple order; the reasons are complex. ... Exceptions to the trend is a function of electron electron interactions ... – PowerPoint PPT presentation
Number of Views:640
Avg rating:3.0/5.0
Title: I' The Aufbau Principle and the Periodic Table
1
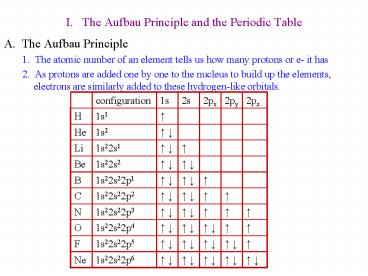
I. The Aufbau Principle and the Periodic Table
- A. The Aufbau Principle
- 1. The atomic number of an element tells us how
many protons or e- it has - 2. As protons are added one by one to the
nucleus to build up the elements, electrons are
similarly added to these hydrogen-like orbitals.
2
- 3. Hunds Rule The lowest energy configuration
for an atom is the one having the maximum number
of unpaired electrons allowed by the Pauli
principle in a particular set of degenerate
orbitals. - 4. We can use the previous Noble Gas as an
abbreviation to indicate filled inner orbitals - a. Na 1s22s22p63s1 or Ne3s1 c. Ca
Ar4s2 - b. Cl Ne3s23p5 d. Rb Kr5s1
3
- Valence Electrons
- Valence electrons are electrons in the outermost
shell only - All other electrons are referred to as core
electrons - Only the valence electrons are involved in
reactivity and bonding - O has 6 valence electrons
- Na has 1 valence electron
- Cl has 7 valence electons
- Elements in the same group (column) of the
periodic table have the same number of valence
electrons (and hence, reactivity and bonding) - Li (2s1), Na(3s1), K(4s1)
- F(2s22p5), Cl(3s23p5), Br(4s24p5)
4
- C. Filling out the Periodic Table
- From energy ordering, 4s fills before 3d
- 3d can hold 10 e- (5 d-orbitals)
- Transition elements have e- in d-orbitals
- Sc Ar4s23d1
- Mn Ar4s23d5
- Zn Ar4s23d10
- Not all transition elements follow simple order
the reasons are complex. You may have to look up
the e- configuration - Cr Ar4s13d5
- Cu Ar4s13d10
5
- D. Broad Periodic Table Classifications
- Representative Elements (main group) fill s and
p orbitals (Na, Al, N) - Transition Elements fill d orbitals (Fe, Co,
Ni) - Lanthanide and Actinide Series (inner transition
elements) fill 4f and 5f orbitals (Eu, Am, Es)
6
IV. Periodic Trends
- Ionization Energy (I.E.) The quantity of energy
required to remove an electron from the gaseous
atom or ion. - X(g) X e-
- It is easiest to take away the first e-, more
energy needed for others - Greater charge makes it harder to extract e-
- First e- comes from the outermost (weakest bound)
orbital - I. E. increases from left to right on periodic
table because larger elements have larger /-
attraction for electrons - I. E. decreases down a group because outer e-
becomes more weakly bound
7
(No Transcript)
8
- Electron Affinity (EA) energy change when e- is
added to a gas atom - X(g) e- -------gt X-(g)
- EA generally become more negative (exothermic)
from left to right - Interactions between the nucleus and the added
electrons is favorable - The larger the nucleus, the better the
interaction - Exceptions to the trend is a function of
electronelectron interactions - N- doesnt form because N has 1s22s22p3
configuration - The extra electron would have to go into an
occupied 2p orbital - C- does form because it has an empty orbital
1s22s22p2 - This phenomenon doesnt always hold true. O- can
form 1s22s22p4 - EA diminishes down a group
- Added e- is farther from the nucleus, so less
interaction is observed - The difference in EA is small and has exceptions
(small F 2p orbital)
9
- C. Atomic Radius half the distance between the
nuclei in a - molecule consisting of identical atoms.
- Easy to understand for diatomic X2 molecules
- Can be calculated from known radii of XY
molecules - Decreases from left to right on periodic table
because /- attraction becomes greater - Increases down a group because more e- and larger
shells filled
10
- Names for groups of the Periodic Table
11
Metals tend to give up electrons to form
cations found on the lower left Nonmetals tend
to gain electrons to become anions found on the
upper right Metalloids have properties of both
metals and nonmetals depending on the conditions