The Quantum Mechanical Model of the Atom - PowerPoint PPT Presentation
1 / 12
Title:
The Quantum Mechanical Model of the Atom
Description:
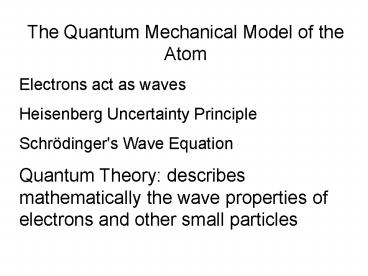
The Quantum Mechanical Model of the Atom Electrons act as waves Heisenberg Uncertainty Principle Schr dinger's Wave Equation Quantum Theory: describes mathematically ... – PowerPoint PPT presentation
Number of Views:116
Avg rating:3.0/5.0
Title: The Quantum Mechanical Model of the Atom
1
The Quantum Mechanical Model of the
Atom Electrons act as waves Heisenberg
Uncertainty Principle Schrödinger's Wave
Equation Quantum Theory describes mathematically
the wave properties of electrons and other small
particles
2
Electrons do not travel around the nucleus in
neat orbits. Orbitals a three dimensional region
around the nucleus that indicates the probable
location of the electron In order to describe
orbitals scientists use quantum numbers Quantum
numbers specify the properties of atomic
orbitals and the properties of electrons in the
orbital
3
- Principle quantum number (n) indicates the
energy level occupied by the electron - n can be 1,2,3
Angular momentum quantum number (l) indicates
the shape of the orbital l is always 0 to
n-1 Each number for l has a letter associated
with it 0s 1p 2d 3f
4
Magnetic quantum number (ml) indicates the
orientation around the nucleus ml is -l to l
What are the quantum numbers if n1 n2 n3 n4
5
(No Transcript)
6
(No Transcript)
7
Spin quantum number (ms) indicates the spin of
an electron in an orbital Has only two options
1/2 or -1/2 Look at all the possible quantum
numbers for n2
8
Electron Configuration The arrangement of the
electrons in the atom
Aufbau Principle an electron occupies the
lowest-energy orbital that can receive it first.
9
(No Transcript)
10
Pauli exclusion Principle no two electrons in
the same atom can have the same set of four
quantum numbers
ms
n
l
ml
11
Hunds rule orbitals of equal energy are each
occupied by one electron before any orbital is
occupied by a second electron, and all electrons
of singly occupied orbitals must have the same
spin.
12
(No Transcript)