Arrhenius PowerPoint PPT Presentations
All Time
Recommended
ACIDS AND BASES Lesson 1 Arrhenius Theory Bronsted-Lowry Theory
| PowerPoint PPT presentation | free to view
Collision Theory. Collisions between two (or more) atoms/molecules required ... Collision theory: reaction occurs only if the reactants collide with a kinetic ...
| PowerPoint PPT presentation | free to download
ACIDOS Y BASES ARRHENIUS ACIDS & BASES ARRHENIUS ACID Un cido Arrhenius es una sustancia que provee iones de hidr geno H+, cuando se disuelve en agua.
| PowerPoint PPT presentation | free to download
And acid is an proton (H ) donor and a base is a proton acceptor. ... The hydroxides of alkaline earths Ca(OH)2 etc. are strong dibasic bases, but ...
| PowerPoint PPT presentation | free to view
HCN/CN- HCl/Cl- NH3/NH4 Acid dissociation constant. Equilibrium expression for ... Amphoteric. Dissociation constant for water. Kw = [H3O ][OH-] = 1.0 x 10-14 ...
| PowerPoint PPT presentation | free to view
Strengths of Acids Arrhenius & Bronsted-Lowry definition: gives H+, hydronium ions in solution Strong acids break down 100% in solution HCl hydrochloric acid
| PowerPoint PPT presentation | free to download
This definition is limited to aqueous solutions. Only one kind of ... Amphoteric Compounds. Compounds that behave as both an acid and a base. Water autoionizes ...
| PowerPoint PPT presentation | free to view
Svante Arrhenius:The First Climate Prediction
| PowerPoint PPT presentation | free to view
Arrhenius acid - any HA, H2A, H3A, or RCOOH substance with an H+ to donate Examples include HCl, H2SO4, H3PO4, and CH3COOH
| PowerPoint PPT presentation | free to view
CH 104: ACID-BASE PROPERTIES OF AQUEOUS SOLUTIONS In the Arrhenius theory an acid produces H+ in aqueous solution and a base produces OH in aqueous solution.
| PowerPoint PPT presentation | free to download
National Centre for Catalysis Research. Indian Institute of ... higher energies that is h? '2pkBT then the expression is similar to M B distribution fucntion. ...
| PowerPoint PPT presentation | free to view
Three models of acids: l. Arrhenius Model Basis for the model--action in water acid definition: produces H in water solution base definition: produces OH1- in water ...
| PowerPoint PPT presentation | free to download
acetic acid base acetate ion water ... The acetic acid produced contributes to the available weak acid. CH3COO H3O CH3COOH H2O ...
| PowerPoint PPT presentation | free to view
The Arrhenius Theory of Acids and Bases Arrhenius acid HCl Arrhenius base NaOH ... Presentation Balance these neutralization equations ...
| PowerPoint PPT presentation | free to view
Chapter 14 Preview Lesson Starter Objectives Acids Bases Arrhenius Acids and Bases
| PowerPoint PPT presentation | free to view
Chapter 15 Acids and Bases Arrhenius Acid-Base Theory Arrhenius, Svante August (1859-1927), Swedish chemist 1903 Nobel Prize in chemistry acid - proton donor base ...
| PowerPoint PPT presentation | free to view
Chapter 14 Preview Lesson Starter Objectives Acids Bases Arrhenius Acids and Bases
| PowerPoint PPT presentation | free to view
ASAM BASA Teori asam basa Arrhenius Tahun 1884 Svante August Arrhenius menyatakan bahwa sifat asam dan basa suatu zat ditentukan oleh jenis ion yg dihasilkan dalam air.
| PowerPoint PPT presentation | free to download
In 1884, Svante Arrhenius proposed these definitions ... Boron trifluoride (a Lewis acid) O. C. H. 3. C. H. 2. C. H. 3. C. H. 2. F. F. O. C. H. 3. C. H. 2. C. H. 3 ...
| PowerPoint PPT presentation | free to view
ARRHENIUS ACIDS. Acids are hydrogen-containing compounds that ionize (dissolve) to yield hydrogen ions H+ in aqueous solutions
| PowerPoint PPT presentation | free to download
Collision Theory * * Reaction Coordinate Diagrams Multistep Reactions Rates, Temperature and Ea Boltzmann Plots and k: Temperature: Activation Energy: Arrhenius ...
| PowerPoint PPT presentation | free to download
Acid-Base Theories OBJECTIVES: Compare and contrast acids and bases as defined by the theories of: a) Arrhenius, b) Br nsted-Lowry, and ...
| PowerPoint PPT presentation | free to download
Collision Theory Reaction Coordinate Diagrams Multistep Reactions Arrhenius Equation: Temperature and Ea Dependence Example 1. Use the data below to find Ea.
| PowerPoint PPT presentation | free to download
ACIDO BASICO Svante Arrhenius, (1859-1927), ACIDO: sustancia que produce iones hidrogeno (H+),al disociarse en agua. Ejemplos HCl ,H2SO4,HNO3, que al ...
| PowerPoint PPT presentation | free to download
Chapter 16 Acids and Bases Some Definitions Arrhenius Acid: Substance that, when dissolved in water, increases the concentration of hydrogen ions.
| PowerPoint PPT presentation | free to view
Acids of Bases Author: whrhs Last ... Strong Acid/Weak Acid Arrhenius Acid Base Concept Bronsted-Lowry Model Bronsted-Lowry Model Lewis Acid/Base ...
| PowerPoint PPT presentation | free to view
Kjemisk reaksjonsteknikk Review of Lecture 1 and 2 (chapter 1 and 2) Rate Laws Reaction Orders Arrhenius Equation Activation Energy Effect of Temperature
| PowerPoint PPT presentation | free to download
Chapter Four Chemical Reactions in Aqueous Solutions Electrostatic Forces Conduction Illustrated Arrhenius s Theory of Electrolytic Dissociation Why do some ...
| PowerPoint PPT presentation | free to download
Review of Acids, Bases, & Salts Arrhenius Acid Has H in the formula Produces H+ as the only positive ion in solution Formula of an Acid Inorganic formula starts ...
| PowerPoint PPT presentation | free to download
Title: ARRHENIUS THEORY ACIDS and BASES Author: ef Last modified by: Administrator Created Date: 7/28/2001 5:01:58 AM Document presentation format
| PowerPoint PPT presentation | free to download
Section 4.3 Acid-Base Reactions Acids: Substances that increase the concentration of H+ when dissolved in water (Arrhenius). Proton donors (Br nsted Lowry).
| PowerPoint PPT presentation | free to download
Acids/Bases Svante Arrhenius, a Swedish chemist, proposed the following definition: Acids form hydronium ions in aqueous solutions, while bases form hydroxide ions.
| PowerPoint PPT presentation | free to view
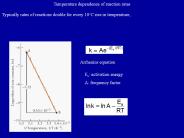
The temperature dependence of the rate of a chemical reaction can be accurately explained by Arrhenius equation. It was first proposed by Dutch chemist, ...
| PowerPoint PPT presentation | free to view
According to Svante Arrhenius concept: Acid is any substance that, when dissolved in water, increase the concentration of hydrogen ion H+.
| PowerPoint PPT presentation | free to view
Chapter 16: Acids and Bases Understand the Arrhenius and Bronsted-Lowry models for acids and bases. Understand acid strength Understand the relationship between acid ...
| PowerPoint PPT presentation | free to download
DEFINITION OF ACIDS AND BASES Christina & Jordan ACIDS AND BASES ARRHENIUS ACIDS: BR NSTED-LOWRY BASES: BR NSTED-LOWRY Amphiprotic Substances WORKS CITED DEFINITION ...
| PowerPoint PPT presentation | free to view
Coppie coniugate acido-base OSSIDI ACIDI, BASICI E ANFOTERI ACIDI E BASI LA NATURA DEGLI ACIDI E DELLE BASI Teoria di Arrhenius: sono acide (basiche) ...
| PowerPoint PPT presentation | free to download
Chapter 16: Acids and Bases Understand the Arrhenius and Bronsted-Lowry models for acids and bases. Understand acid strength Understand the relationship between acid ...
| PowerPoint PPT presentation | free to download
The Effects of Temperature and Catalyst on Reaction Rate 15 15.1 Activation Energy and Arrhenius Equation 15.2 Interpretation of Rates of Gaseous
| PowerPoint PPT presentation | free to view
Introduction to Acids and Bases The earliest definition was given by Arrhenius: An acid contains a hydrogen atom and dissolves in water to form a hydrogen ion, H+.
| PowerPoint PPT presentation | free to view
Acids and Bases! Today s Objectives Students will be able to State and apply the Arrhenius Theory of Acids and Bases. Interpret the pH scale and use it to ...
| PowerPoint PPT presentation | free to view
Selection of Aluminium Electrolytic Capacitors to match lighting applications ... X-Ray Image. Safety Vent. Electrolyte dissipation follows the Arrhenius Rate ...
| PowerPoint PPT presentation | free to view
Modern Theories of Acids & Bases The Arrhenius and Bronsted-Lowry Theories Go to pH- Indicator PowerPoint Go to pH- Indicator PowerPoint Types of Salts Salts can be ...
| PowerPoint PPT presentation | free to view
Brickwork layer model shows Cgb Cb ... Unpolished. Polished. RT ~ Rgb = 2.04 kW. Cgb = 7.5 nF. Both Rb and Rgb obey the Arrhenius law. ...
| PowerPoint PPT presentation | free to download
Chapter 18 Equilibria Involving Acids & bases ARRHENIUS THEORY for ACIDS and BASES ACIDS: produce hydrogen ions (protons), H+, in solution BASES: produce hydroxide ...
| PowerPoint PPT presentation | free to download
59. Which ion is the only negative ion produced by an Arrhenius base in water? (1) NO3-1 (2) Cl 1 (3) OH 1 (4) H 1 60. When the elements in Group 1 are ...
| PowerPoint PPT presentation | free to download
It is also used in car batteries ... hydroxide Arrhenius Acids & Bases An Arrhenius acid is a chemical compound that increases the concentration of hydrogen, H+ ...
| PowerPoint PPT presentation | free to download
Catalysis Homogeneous Catalysis Catalysts can operate by increasing the number of effective collisions. That is, from the Arrhenius equation: catalysts increase k be ...
| PowerPoint PPT presentation | free to download
Properties of acid and base solutions. Arrange acids according to acid strength ... Taste. Arrhenius theory of acids/bases. Developed in 1884. ...
| PowerPoint PPT presentation | free to download
Una Nueva Teor a Acido - Base Las definiciones de Arrhenius de los cidos y bases son muy tiles en el caso de las soluciones acuosas, ...
| PowerPoint PPT presentation | free to download
Chapter Fifteen Acids, Bases, and Acid Base Equilibria The Br nsted Lowry Theory Arrhenius theory: an acid forms H+ in water; and a base forms OH in water.
| PowerPoint PPT presentation | free to download
'Structural Investigation and Hydrogen Storage Capacity of New Intermetallic Compounds' ... aDivision of Structural Chemistry, Arrhenius Laboratory, Stockholm ...
| PowerPoint PPT presentation | free to view
If the hydroxide ion concentration is 10-10 M, what is the pH of the solution? ... Lowry base, but not an Arrhenius base: a) sodium hydroxide, or b) ammonia? ...
| PowerPoint PPT presentation | free to view
Arrhenius equation and transition state theory ... to the concentration of reactants raised ... The reactants must engage in an encounter (collision) event. ...
| PowerPoint PPT presentation | free to view
restores vegetable colors reddened by acids. react with acids ... litmus, phenolphthalein, thymol blue. Arrhenius Acid: substance that releases or produces H ...
| PowerPoint PPT presentation | free to view
Shear strain; e=s/G0, strain rate; de/dt=s/tG0, so that viscosity; h~tG0 ... As a result we expect viscosity to be Arrhenius. ... Both F&G goes to a minimum at ...
| PowerPoint PPT presentation | free to download