Arrhenius equation - PowerPoint PPT Presentation
Title:
Arrhenius equation
Description:
Collision Theory. Collisions between two (or more) atoms/molecules required ... Collision theory: reaction occurs only if the reactants collide with a kinetic ... – PowerPoint PPT presentation
Number of Views:2246
Avg rating:3.0/5.0
Title: Arrhenius equation
1
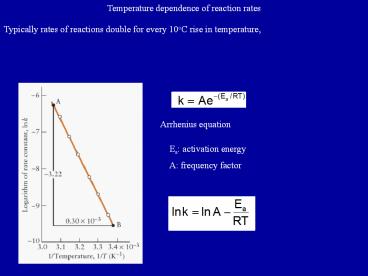
Temperature dependence of reaction rates
Typically rates of reactions double for every
10oC rise in temperature,
Arrhenius equation
Ea activation energy A frequency factor
2
An Arrhenius plot of ln k against 1/T is used to
determine Ea and A The higher the Ea the
stronger the temperature dependence of the rate
constant
3
Collision Theory
- Collisions between two (or more) atoms/molecules
required for a reaction. - However, every time two reactants collide they
may not react - As temperature increases
- atoms/molecules collide more frequently
- kinetic energy of atoms/molecules increases
- Collision theory reaction occurs only if the
reactants collide with a kinetic energy of at
least the activation energy, and they do so in
the correct orientation.
4
Kinetic energy is important
5
(No Transcript)
6
Orientation is important
Cl
N
O
2 AB -gt A2 B2
2 NOCl ? 2 NO Cl2
7
Animation 1
Animation 2
Animation 3
8
The factor e-Ea/RT fraction of molecules that
have at least the minimum energy required for
reaction. For an Ea 40 kJ/mol Temperature (K)
e-Ea/RT 298 9.7 x 10-8 400 5.9 x
10-6 600 3.3 x 10-4 A reflects orientation
effect or steric effect
9
Measuring k as a function of T Ea to be determined
10
Reaction coordinate diagram Activated complex or
transition state - highest energy along reaction
coordinate Reactants must collide with sufficient
energy to reach this point and collide in a
preferred orientation to form the activated
complex
11
DE (Ea)forward - (Ea)reverse
12
Higher temperatures favor products for an
endothermic reaction and reactants for an
exothermic reaction
Endothermic reaction Ea(forward) gt
Ea(reverse) Exothermic reaction Ea(forward) lt
Ea(reverse)
13
CH3OH(aq) H(aq) ? CH3OH2(aq) CH3OH2(aq)
Br- (aq) ? CH3Br H2O(aq)
14
Catalysis
Catalyst a compound which speeds up the rate of
a reaction, but does not itself undergo a
chemical change. Simple mechanism A catalyst
? intermediates intermediates ? B
catalyst Overall A ? B Concentration of
catalyst is included in k hence k varies with
concentration of catalyst
15
Presence of a catalyst provides an alternate path
with a lower Ea
2H2O2(aq) ? 2H2O(aq) O2(g) In the absence of a
catalyst, Ea 76 kJ/mol In the presence of
a catalyst (I-) Ea 57 kJ/mol rate
constant increases by a factor of 2000
16
Catalyzed by I2
17
Pt
C2H4(g) H2(g) ? C2H6 (g)
Example of heterogenous catalysis
18
A catalyst does not effect the thermodynamics of
the reaction
DG is not affected by catalyst neither is
K Equilibrium concentrations are the same with
and without catalyst just the rate at which
equilibrium is reached increases in the presence
of a catalyst
K k1/k-1 catalyst speeds up both the forward
and reverse reaction
19
Enzymes Practically all living reactions are
catalyzed by enzymes each enzyme specific for a
reaction. Enzymes typically speed up rates by 107
- 1014 times rate of uncatalyzed reactions Ea for
acid hydrolysis of sucrose 107 kJ/mol Ea for
catalyzed acid hydrolysis of sucrose 36
kJ/mol Rate increase of 1012 at body temperature
E S ? ES ES ? P E
20
Poisoning a catalyst Arsenic poisoning
Ingestion of As(V) as AsO43- results in reduction
to As(III) which binds to enzymes, inhibiting
their action Nerve gases - block
enzyme-controlled reactions that allow nerve
impulses to travel through the nerves.
21
Catalytic Converters Incomplete combustion of
gasoline produces CO, hydrocarbon fragments
(CmHn) High temperature in the engine causes
oxidation of N2 to NO and NO2 Conversion of
these pollutants to less harmful compounds is
speeded up in the presence of catalysts.
Catalyst pellets of Pt, Pd, Rh
animation
22
(No Transcript)