Introduction to Acids and Bases - PowerPoint PPT Presentation
1 / 38
Title:
Introduction to Acids and Bases
Description:
Introduction to Acids and Bases The earliest definition was given by Arrhenius: An acid contains a hydrogen atom and dissolves in water to form a hydrogen ion, H+. – PowerPoint PPT presentation
Number of Views:90
Avg rating:3.0/5.0
Title: Introduction to Acids and Bases
1
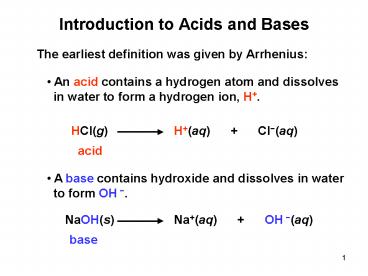
Introduction to Acids and Bases
The earliest definition was given by Arrhenius
- An acid contains a hydrogen atom and dissolves
- in water to form a hydrogen ion, H.
HCl(g)
H(aq) Cl-(aq)
acid
- A base contains hydroxide and dissolves in water
- to form OH -.
NaOH(s)
Na(aq) OH -(aq)
base
2
Introduction to Acids and Bases
- The Arrhenius definition correctly predicts the
- behavior of many acids and bases.
- However, this definition is limited and
sometimes - inaccurate.
- For example, H does not exist in water.
Instead, it - reacts with water to form the hydronium ion,
H3O.
H3O(aq)
H(aq) H2O(l)
hydronium ion actually present in aqueous
solution
hydrogen ion does not really exist in solution
3
Introduction to Acids and Bases
The BrønstedLowry definition is more widely used
- A BrønstedLowry acid is a proton (H) donor.
- A BrønstedLowry base is a proton (H) acceptor.
This proton is donated.
H3O(aq) Cl-(aq)
HCl(g) H2O(l)
- HCl is a BrønstedLowry acid because it donates
- a proton to the solvent water.
- H2O is a BrønstedLowry base because it accepts
- a proton from HCl.
4
Introduction to Acids and Bases BrønstedLowry
Acids
- A BrønstedLowry acid must contain a hydrogen
- atom.
- Common BrønstedLowry acids (HA)
HCl hydrochloric acid
H2SO4 sulfuric acid
HBr hydrobromic acid
HNO3 nitric acid
5
Introduction to Acids and Bases BrønstedLowry
Bases
- A BrønstedLowry base is a proton acceptor,
- so it must be able to form a bond to a proton.
- A base must contain a lone pair of electrons
that - can be used to form a new bond to the proton.
This e- pair forms a new bond to a H from H2O.
H
H2O(l)
H
N
H
H
N
H
OH -(aq)
H
H
BrønstedLowry base
6
Introduction to Acids and Bases BrønstedLowry
Bases
- Common BrønstedLowry Bases(B )
Lone pairs make these neutral compounds bases.
The OH - is the base in each metal salt.
NaOH sodium hydroxide
KOH potassium hydroxide
Mg(OH)2 magnesium hydroxide
Ca(OH)2 calcium hydroxide
7
Proton Transfer The Reaction of a BrønstedLowry
Acid witha BrønstedLowry Base
This e- pair forms a new bond to H.
This e- pair stays on A.
gain of H
H
A
H
B
acid
base
loss of H
8
Proton Transfer The Reaction of a BrønstedLowry
Acid witha BrønstedLowry Base
gain of H
H
A
H
B
conjugate acid
acid
base
conjugate base
loss of H
- The product formed by loss of a proton from an
- acid is called its conjugate base.
- The product formed by gain of a proton by a base
- is called its conjugate acid.
9
Proton Transfer The Reaction of a BrønstedLowry
Acid witha BrønstedLowry Base
gain of H
H2O
H
Br
Br-
H3O
conjugate acid
acid
base
conjugate base
loss of H
- HBr and Br- are a conjugate acidbase pair.
- H2O and H3O are a conjugate acidbase pair.
- The net charge must be the same on both sides
- of the equation.
10
Proton Transfer The Reaction of a BrønstedLowry
Acid witha BrønstedLowry Base
Amphoteric compound A compound that contains
both a hydrogen atom and a lone pair of e- it
can be either an acid or a base.
H
add H
H
O
H
H
O
H
H2O as a base
conjugate acid
-
remove H
H
O
H
H
O
H2O as an acid
conjugate base
11
Acid and Base StrengthRelating Acid and Base
Strength
- When an acid dissolves in water, the proton
- transfer that forms H3O is called dissociation.
- When a strong acid dissolves in water, 100 of
- the acid dissociates into ions.
H3O(aq) Cl-(aq)
HCl(g) H2O(l)
- A single reaction arrow is used, because the
- product is greatly favored at equilibrium.
- Common strong acids are HI, HBr, HCl, H2SO4,
- and HNO3.
12
Acid and Base StrengthRelating Acid and Base
Strength
- When a weak acid dissolves in water, only a
- small fraction of the acid dissociates into
ions.
- Unequal reaction arrows are used, because the
reactants are usually favored at equilibrium.
H3O(aq) CH3COO-(aq)
CH3COOH(l) H2O(l)
13
Acid and Base StrengthRelating Acid and Base
Strength
- When a strong base dissolves in water, 100 of
- the base dissociates into ions.
Na(aq) OH-(aq)
NaOH(s) H2O(l)
- Common strong bases are NaOH and KOH.
- When a weak base dissolves in water, only a
- small fraction of the base dissociates into
ions.
NH4(aq) OH-(aq)
NH3(g) H2O(l)
14
Acid and Base StrengthRelating Acid and Base
Strength
- A strong acid readily donates a proton, forming
- a weak conjugate base.
HCl strong acid
Cl- weak conjugate base
- A strong base readily accepts a proton, forming
- a weak conjugate acid.
OH- strong base
H2O weak conjugate acid
15
Dissociation of Water
Water can behave as both a BrønstedLowry
acid and a BrønstedLowry base. Thus, two water
molecules can react together in an acidbase
reaction
loss of H
H
H
O
H
H
O
H
H
O
H
base
acid
conjugate acid
conjugate base
gain of H
16
Dissociation of Water
- Pure water contains an exceedingly low
- concentration of ions, H3O and OH. Since one
- H3O ion and one OH ion are formed in each
- reaction, their concentrations are equal in
pure - water.
H3O OH- 1.0 107 M at 25 C.
- Multiplying these concentrations together gives
the - ion-product constant for water, Kw.
Kw H3OOH-
ion-product constant
17
Dissociation of Water
- Substituting the concentrations for H3O and OH
- into the expression for Kw gives the following
- result.
Kw H3O OH-
Kw (1.0 x 10-7) x (1.0 x 10-7)
Kw 1.0 x 10-14
- Kw is a constant, 1.0 x 10-14, for all aqueous
- solutions at 25 oC.
18
Dissociation of Water
To calculate H3O when -OH is known
To calculate -OH when H3O is known
Kw H3OOH-
Kw H3OOH-
Kw
Kw
OH-
H3O
OH-
H3O
1 x 10-14
1 x 10-14
OH-
H3O
H3O
OH-
19
Dissociation of Water
If the H3O in a cup of coffee is 1.0 x 10-5 M,
then the -OH can be calculated as follows
Kw
1 x 10-14
OH-
1.0 x 10-9 M
H3O
1 x 10-5
In this cup of coffee, therefore, H3O gt OH-,
and the solution is acidic overall.
20
Dissociation of Water
21
The pH ScaleCalculating pH
pH -log H3O
The lower the pH, the higher the concentration of
H3O.
- Acidic solution pH lt 7 ? H3O gt
1 x 10-7
- Neutral solution pH 7 ? H3O 1
x 10-7
- Basic solution pH gt 7 ? H3O lt
1 x 10-7
22
The pH ScaleCalculating pH from H3O
- If H3O 1.0 x 105 M for a urine sample,
- what is its pH?
pH log H3O log (1.0 x 105)
(5.00) 5.00
- The urine sample is acidic because the pH lt 7.
23
The pH ScaleCalculating H3O from pH
- If the pH of seawater is 8.50, what is the
H3O?
pH -log H3O
8.50 -log H3O
-8.50 log H3O
antilog (-8.50 ) H3O
H3O 3.2 x 10-9 M
- The seawater is basic because H3O gt 1 x 107
M.
24
The pH ScaleCalculating pH
- A logarithm has the same number of digits to the
- right of the decimal point as are contained in
the - coefficient of the original number.
H3O 3.2 x 10-9 M
pH 8.50
pH 8.50
two significant figures
two digits after decimal point
25
Focus on the Human BodyThe pH of Body Fluids
26
Common AcidBase ReactionsReaction of Acids with
Hydroxide Bases
Neutralization reaction An acid-base reaction
that produces a salt and water as products.
HA(aq) MOH(aq)
H
OH(l) MA(aq)
base
water
salt
acid
- The acid HA donates a proton (H) to the OH-
base - to form H2O.
- The anion A- from the acid combines with the
- cation M from the base to form the salt MA.
27
Common AcidBase Reactions
HOW TO Draw a Balanced Equation for a
Neutralization Reaction Between HA and MOH
Write a balanced equation for the reaction of
Mg(OH)2 with HCl.
Example
Identify the acid and base in the reactants and
draw H2O as one product.
Step 1
H2O(l)
salt
HCl(aq) Mg(OH)2(aq)
water
base
acid
28
Common AcidBase Reactions
HOW TO Draw a Balanced Equation for a
Neutralization Reaction between HA and MOH
Step 2
Determine the structure of the salt.
- The salt is formed from the parts of the acid
and - base that are not used to form H2O.
HCl
Mg(OH)2
H reacts to form H2O
Cl- used to form salt
Mg2 used to form salt
2 OH- react to form water
Mg2 and Cl- combine to form MgCl2.
29
Common AcidBase Reactions
HOW TO Draw a Balanced Equation for a
Neutralization Reaction between HA and MOH
Step 3
Balance the equation.
Place a 2 to balance O and H.
2
H2O(l)
MgCl2
2
HCl(aq) Mg(OH)2(aq)
acid
base
water
salt
Place a 2 to balance Cl.
30
Common AcidBase ReactionsReaction of Acids with
Hydroxide Bases
A net ionic equation contains only the species
involved in a reaction.
HCl(aq) NaOH(aq)
HOH(l) NaCl(aq)
- Written as individual ions
H(aq) Cl-(aq) Na(aq) OH-(aq)
HOH(l) Na(aq) Cl-(aq)
- Omit the spectator ions, Na and Cl.
- What remains is the net ionic equation
H(aq) -OH(aq)
HOH(l)
31
Common AcidBase ReactionsReaction of Acids with
Bicarbonate Bases
- A bicarbonate base, HCO3-, reacts with one H to
- form carbonic acid, H2CO3.
H(aq) HCO3-(aq)
H2CO3(aq)
H2O(l) CO2(g)
- Carbonic acid then decomposes into H2O and CO2.
- For example
HCl(aq) NaHCO3(aq)
NaCl(aq) H2CO3(aq)
H2O(l) CO2(g)
32
Common AcidBase ReactionsReaction of Acids with
Bicarbonate Bases
- A carbonate base, CO32, reacts with two H to
- form carbonic acid, H2CO3.
2 H(aq) CO32(aq)
H2CO3(aq)
H2O(l) CO2(g)
- For example
2 HCl(aq) CaCO3(aq)
2 CaCl2(aq) H2CO3(aq)
H2O(l) CO2(g)
33
Buffers
A buffer is a solution whose pH changes very
little when acid or base is added.
Most buffers are solutions composed of
roughly equal amounts of
- a weak acid.
- the salt of its conjugate base.
The buffer resists change in pH because
- added base, -OH, reacts with the weak acid.
- added acid, H3O, reacts with the conjugate
base.
34
BuffersGeneral Characteristics of a Buffer
If an acid is added to the following buffer
equilibrium,
Adding more product
CH3COOH(aq) H2O(l)
H3O(aq) CH3COO-(aq)
conjugate base
weak acid
drives the reaction to the left.
then the excess acid reacts with the conjugate
base, so the overall pH does not change much.
35
BuffersGeneral Characteristics of a Buffer
If a base is added to the following buffer
equilibrium,
Adding more reactant
H2O(l) CH3COO-(aq)
CH3COOH(aq) -OH(aq)
conjugate base
weak acid
drives the reaction to the right.
then the excess base reacts with the weak acid,
so the overall pH does not change much.
36
Focus on the Human BodyBuffers in the Blood
- Normal blood pH is between 7.35 and 7.45.
- The principle buffer in the blood is carbonic
acid/ - bicarbonate (H2CO3/HCO3-).
H2O
H2CO3(aq)
CO2(g) H2O(l)
H3O(aq) HCO3-(aq)
- CO2 is constantly produced by metabolic
- processes in the body.
- The amount of CO2 is related to the pH of the
blood.
37
Focus on the Human BodyBuffers in the Blood
Respiratory acidosis results when the body fails
to eliminate enough CO2, due to lung disease or
failure.
A lower respiratory rate increases CO2.
H3O(aq) HCO3-(aq)
CO2(g) 2 H2O(g)
This drives the reaction to the right, increasing
H3O.
Blood then has a higher H3O and a lower pH.
38
Focus on the Human BodyBuffers in the Blood
Respiratory alkalosis is caused by
hyperventilating very little CO2 is produced by
the body.
A higher respiratory rate decreases CO2.
H3O(aq) HCO3-(aq)
CO2(g) 2 H2O(g)
This drives the reaction to the left, decreasing
H3O.
Blood then has a lower H3O and a higher pH.