PowerPoint-Pr - PowerPoint PPT Presentation
1 / 87
Title:
PowerPoint-Pr
Description:
HOW TO DETERMINE PHYTOPLANKTON? Silvana V. Rodrigues Determination of phytoplynkton composition and biovolume Uterm hl method:: Advantage: asy sampling, long storage ... – PowerPoint PPT presentation
Number of Views:706
Avg rating:3.0/5.0
Title: PowerPoint-Pr
1
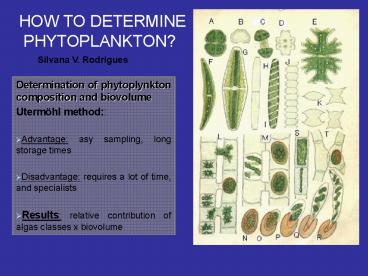
HOW TO DETERMINE PHYTOPLANKTON?
Silvana V. Rodrigues
- Determination of phytoplynkton composition and
biovolume - Utermöhl method
- Advantage asy sampling, long storage times
- Disadvantage requires a lot of time, and
specialists - Results relative contribution of algas classes x
biovolume
2
HOW TO DETERMINE PHYTOPLANKTON ?
peridinina
Dinoflagelados
Clorophyta
Cryptophyta
Cyanobacterias
aloxanthin
3
http //oceancolor.gsfc.nasa.gov/.../BIOLOGY/
4
Importance of chlorophyll a
- 1.000 milhão tons produzidas por ano na terra e
no mar - indicator único da biomassa aquática
- parâmetro bioquímico mais freqüentemente medido
- em oceanografia
Cloroplasto
fig.cox.miami.edu/.../phts/c8.10x21.overview.jpg
struggle.net/history/images/ molecule.jpgwww.molec
ularexpressions.com
5
Function of pigments in photosynthetic organisms
- chlorophyll a
- light absorption (Light harvesting complexes)
- electron donor and acceptor in reative centers
- Carotenoids
- Light absorption
- Protection of chlorophyll (quenching of Chl
photoinduced triplet - state ) and quenching of O2 singlet state .
6
divisão/classe nome comum gên espéc.
Algas marrons(clorofilas a e c) Algas marrons(clorofilas a e c) Algas marrons(clorofilas a e c) Algas marrons(clorofilas a e c)
Bacillariophyta diatomáceas 210 Desconh.
Dinophyta dinoflagelados 550 4000
Crysophyta Chrysophyceae Rapidophyceae flagelados marrom-amarel. Crysophytas,silicoflagelados raphydophytas (cloromonadas) 120 4 1000 9
Haptophyta Primnesiophyceae flagelados marrom-amarel. cocolitoforídeos 50 500
Xantophyta algas verde-amareladas 50 600
Cryptophyta criptomonadas 8 gt50
Eustigmatophyta algas amarelo-esverdeadas 6 12
Algas verdes (clorofilas a e b) Algas verdes (clorofilas a e b) Algas verdes (clorofilas a e b) Algas verdes (clorofilas a e b)
Chlorophyta Clorophyceae Prasinophyceae Euglenophyta Algas verdes Flagelados verdes Euglenoides 350 13 43 2500 120 650-800
Algas vermelhas (clorofila a e biliproteínas) Algas vermelhas (clorofila a e biliproteínas) Algas vermelhas (clorofila a e biliproteínas) Algas vermelhas (clorofila a e biliproteínas)
Rhodophyta Algas vermelhas 3 10
Algas azuis (Cyanobacteria) ( clorofila a e biliproteínas) Algas azuis (Cyanobacteria) ( clorofila a e biliproteínas) Algas azuis (Cyanobacteria) ( clorofila a e biliproteínas) Algas azuis (Cyanobacteria) ( clorofila a e biliproteínas)
Cyanophyta Prochlorophyta Cianobactérias proclorofitas
7
Characteristics which make it possible to use
algal pigments (chlorophylls, carotenoids and
phycobiliproteins) as chemotaxonomic markers
- They are present in all photosynthetic algae,
but absent in most bacteria, - protozoa and detritus
- Many occur only in specific classes or even
genera, allowing the - determination of phytoplankton taxonomic
composition at least at class level, - or better
- They are strongly coloured, and in the case of
chlorophylls and phycobiliproteins - are fluorescent, what allows their detection with
high sensitivity, - Most of them are labile and esily dgraded after
cell death, allowing to - distinguish living from dead cells
8
Hystorical overview
- 1952 chlorophyll was recognized as a selective
phytoplankton marker, in the presence of other
biological components (zooplankton, bacteria,
detritus) - 1984-1987 HPLC methods for the determination of
chls, carotenoids and phytoplankton degradation
products
- Use of pigment chemotaxonomy for recognition, in
field samples, of phytoplanktonic classes not
detected since then, because of preservation
problems or filtration losses. - alloxanthin (Cryptophyta)
- chlor b (Chlorophyta and Prasinophyta)
- zeaxanthin (Cyanobacteria)
- 19-hexanoiloxifucoxanthin (Prymnesiophyta)
- divynil-chlorophyill a (Proclorophyta)
9
Chlorophylls 132 -Metilcarboxilates
of - Mg-phytoporphyrin (double bond in D ring)
Cl c, Mg-phytoclhorin Cl a, Cl b
Phytil at C-173 (Cl a and b)
Propionic acid at C17 Cl a and b
Acrílic acid at C17 Cl c
Mg coordination complexes with cyclic
tetra-pyrrols Macrocicles with five member rings
10
Chlorophylls 132 -Metilcarboxilates
of - Mg-phytoporphyrin (double bond in D ring)
Cl c, Mg-phytoclhorin Cl a, Cl b
Phytil at C-173 (Cl a and b)
Propionic acid at C17 Cl a and b
Acrílic acid at C17 Cl c
Oxo substituent at C-131 methyl-carboxilate
groups at C-132 -
11
chlorophyll b
chlorophyll a
DV-chlorophyll a
DV-chlorophyll b
Molecule drawingsN. Montoya
12
chlorophyll c1
chlorophyll c2
chlorophyll c3
Molecule drawingsN. Montoya
13
Degradation by chemical processes
Molecules become chemically and fotochemically
more labile in organic solvents than in the
cells
- Loss of metal
- Chla ? Phaeophitin
- in organic solvents
- In dilute acids
- under high intensity of light
14
Degradation by chemical processes
- Epimerization
- (HPLC in SiO2)
- Allomerization
- (oxidation by O2)
Cl ? enolate ? Cla, b
- Chl a ? 132 Hydroxiclhorophyll a
- Chl a ? Cl a - Hyidroxilactone.
Both processes can be minimized by decreasing
the temperature
In alcoholic or hydro-alcoholic solutions
Specially in pH gt7
15
Degradation by chemical processes
Loss of phytil group Cl ? chlorophyillide
In methanol or ethanol in basic medium
16
Biodegradation
Loss of metal Mg-dequelatase Formation of
phaeophytins
- To cyclic tetra-pirrols
- perifercally modified
- (enzymatically,
- Specially in the absence of
- light and O2)
Decarboximetilation Formation of pirophaeophytins
e pirophaeophorbides
Hydrolisis of the phytil ester
(chlorophyllase) chlorophillide formation
Allomerization Epimerization (Chl-oxidase)
17
Biodegradation
- To linear tetra
- pirrols
5
4
- Normally by oxidative opening
- of the macrocycle ring, between
- C-4 and C-5,
- C-5 stays as an aldehyde
18
Carotenoids
Derive from carotene
C40H56
ß- ß- carotene
Isoprenoid units
Polyen Absorbtion of light. COLOUR
?-carotene ?,?-carotene ?-carotene
?,?-carotene ?-carotene ?,?-carotene ?-carotene
?,?-carotene lycopene ?,?-carotene
19
Properties More stable in phytoplankton and
in plants than chlorophylls they dont have N,
so cant be used in enzymatic amino-acid building.
Example Leaves lose the green colour in autumn
(chlorophyll), But dont lose colours due to
carotenoids
20
Polyene chain is responsible for instability
- Oxidation by air or peroxides
- Electrophyle addition ( H and Lewis acids)
- Isomerization E/Z caused by heat, light or
chemicals, - Undergo reactions at the ends of the molecules
- Production of artefacts
21
Acetil-CoA
Geranylgeranyldiphosphate
Geranylgeranyldiphosphate
Biosynthesis occurs in thylakoid membranes
Phytoene
Dessaturation
Lycopene
Ciclization
?, ? -carotene
?, ? -carotene
Hydroxilation
Hydroxilation
lutein
Zeaxanthin
Deepoxidation
Epoxidation
Dark
Light
Anteraxanthin
Can occur in the dark Depends a lot on light
Epoxidation
Dark
Light
Deepoxidation
Violaxanthin
VIOLAXANTHIN CICLE
Rearrangement
Neoxanthin
22
DIADINOXANTHIN CICLE
Diadinoxantin
epoxidation
LIGHT
DARK
2H - H2O
2H O2 - H2O
Diatoxanthin
23
Carotenoids
C40H56
ß- ß- carotene
Aldehydes, ketones
Enzimatic hydroxilation
Acetates (OCOMe) e lactones
Carboxi (CO2H), carbometoxi (CO2Me) ou metoxi
(OMe)
Hydroxi- carotenoids as fatty acid esters, or
as Glycosides or glycosylesters, others as
sulphates
Epoxidation
24
Xantophylls
Isoprenoids
Zeaxanthin
isomers
Lutein
25
Acetilenic
Diatoxanthin
Alenic
fucoxanthin
Norcarotenoids ( skeleton C37)
Peridinin C39H50O7
26
In acid medium Epoxides rearrange (5,6 to 5,8
form)
7
6
8
5
violaxanthin
7
6
8
5
neoxanthin
27
- In basic medium
- In general stable
- exception
- esters are hydrolysed
- some compounds suffer structural change
(fucoxanthin, peridinin)
fucoxanthin
28
Distribution of chlorophylls among
divisions/classes of phytoplankton
Division or class / Pigment Cyanophyta Prochlorophyta Rhodophyta Cryptophyta Chlorophyceae Prasinophyceae Euglenophyta Eustimatophyta Bacillariophyta Dinophyta Prymnesiophyceae Chrysophyceae Raphidophyceae
Chl a
Chlb
Chl c1
Chl c2
Chl c3
Tipo pyhtilat. Chlc
MgDVP
DVchla
DVChlb
29
Distribution of carotenes among divisions/classes
of phytoplankton
Division or class / Pigment Cyanophyta Prochlorophyta Rhodophyta Cryptophyta Chlorophyceae Prasinophyceae Euglenophyta Eustimatophyta Bacillariophyta Dinophyta Prymnesiophyceae Chrysophyceae Raphidophyceae
?,?
?,?
?,?
?,?
?,?
30
Distribution of xantophylls among
divisions/classes of phytoplankton
Division or class / Pigment Cyanophyta Prochlorophyta Rhodophyta Cryptophyta Chlorophyceae Prasinophyceae Euglenophyta Eustimatophyta Bacillariophyta Dinophyta Prymnesiophyceae Chrysophyceae Raphidophyceae
Aloxanthin
Anteraxanthin
Astaxanthin 2 2 2
19-Butanoil- fucoxanthin
Cantaxanthin 2
Crocoxanthin
Diadinoxanthin
Diatoxanthin
Dinoxanthin
Echinenona 2 2
Fucoxanthin 1
31
Distribution of xantophylls among
divisions/classes of phytoplankton
Division or class / Pigment Cyanophyta Prochlorophyta Rhodophyta Cryptophyta Chlorophyceae Prasinophyceae Euglenophyta Eustimatophyta Bacillariophyta Dinophyta Prymnesiop. Chrysophyceae Raphidophyceae
19hexanoilfuco 1
Luteína
Monadoxanthin
Neoxanthin
P457P468
Peridinina
Peridininol
Prasinoxanthin
Pirroxanthin
Sifonaxanthin 14 14
Sifoneina
Ést. Vaucheriax
Violaxanthin
Zeaxanthin
32
Amphidinium carterae (Dinophyta)
Rzi lpigmi/chlorophyll a
Rz peridinin/chlorophyll a
chlorophyll c2
chlorophyll a
dinoxanthin
peridinin
diadinoxanthin
33
Dunaliella tertiolecta (Chlorophyta)
Rzi lpigmi/chlorophyll a
Rz lutein/chlorophyll a
chlorophyll b
chlorophyll a
neoxanthin
violaxanthin
lutein
anteraxanthin
34
Hierarchical guide to the use of pigments
S. Wright, Class notes
35
Retention times and mean absorption properties
(inHPLC eluant) of the major pigments detected in
Erythrobacter longus (ATCC 33941) and isolates
NAP1, MG3, and NJ3Y. Peak numbers correspond to
those indicated in Fig. 5. Solvents and
caroteneid band ratios from the literature data
1 solventmethanol water (41) containing 40mM
NH4OH, (III/II)0 2 solvent methanol,
(III/II)0 3 solventacetone, (III/II)33 4,
5 solventdiethyl ether 6 solventacetone,
(III/II)21
Peak no. Rt Pigment identification Observed ?max Published ?max Reference
(min) (nm) (nm)
1 11.4 Erythroxanthin sulfate 465 469 Takaichi et al. (1991)
2 18.4 Bacteriorubixanthinal 513 510 Takaichi et al. (1988)
3 19.1 Zeaxanthin (428), 454, 482 (428), 454, 481 Jeffrey et al. (1997)
4 20.4 Bacteriochlorophyll a 359, 580, 771 358, 577, 773 Scheer (1991)
5 23.4 Bacteriophaeophytin a 358, 525, 750 357, 525, 749 Scheer (1991)
6 25.4 ß,ß-carotene (426), 454, 478 (426), 454, 480 Jeffrey et al. (1997)
Michal Kobližek Arch Microbiol (2003) 180
327338
36
Reverse-phase HPLC chromatograms (360 nm)
for acetone extracts prepared from whole cell
pellets of a Erythrobacter longus ATCC 33941, b
NAP1, c MG3, and d NJ3Y. Peak identities 1
erythroxanthin sulfate, 2 bacteriorubixanthinal, 3
zeaxanthin, 4 bacteriochlorophyll a, 5
bacteriophaeophytin a, and 6 ß,ß-carotene
Michal Kobližek Arch Microbiol (2003) 180
327338
37
HPLC chromatogram of fuorescent pigments from a
surface sample (2 m depth) collected at station
C354-004. Excitation was at 365 nm, emission at
780 nm, with 20-nm slits. These wavelengths were
chosen to maximize the signal from BChla, while
minimizing the signal from the more abundant
pigments, Chla and Chlb. (Inset) Fluorescence
emission spectrum of the peak eluting at 16.7 min
in (A). Excitation was at 365 nm and slits
were 20 nm.
Zbigniew S. Kolber et al, Science 292, 2492-2495
2001.
38
PIGMENTS IN SEDIMENTS
39
Pigmentos Em geral são moléculas lábeis, atingem
o sedimento em vários estágios de degradação.
Degradação dos pigmentos originais
principalmente na água e na superfície do
sedimento, durante a deposição (Hodgson et al.,
1997)
- Na água
- rápida e extensa
- (95 dos compostos em poucos dias)
- digestão por herbívoros,
- enzimática, na senescência celular
- oxidação química, microbiológica e pela luz.
Fatores que afetam a taxa de degradação
- Tempo para chegar
- ao fundo
- Tipo de pigmento
- Grau de ataque
- químico e biológico
- Nos sedimentos
- taxa de degradação menor, especialmente em
condições anóxicas. Depende de - intensidade de luz e da
- bioturvação invertebrada
40
- DEGRADATIN PRODUCTS
- degradation to uncoloured compounds
- conversion to cis-carotenoids and phaeopigments
more difficult to identify (Steenbergen et al.,
1994 apud Hodgson et al., 1997).
Separation and quantification of pigments in
sediments More complex than in phytoplankton
samples, due to the variety of degradation or
transformation products (Mendes et al. 2007) .
41
Chlorophyll b occurs mainly ingreen algae and
vascular plants, Chlorophylls c in diatoms,
dinophlagellates and some brown algae
Kowalewska et al., 2004.
Chl a and phaeophytin degradação products due
to Environmental stress
Pirophaeophitins and steril Chlorins
degradation products due to zooplankton
Phaeophorbides Degradation products due to
zooplankton
Jeffrey, 1997 apud Kowalewska et al., 2004).
42
Fossile Pigments Used in paleoclimatic and
paleoenvironmental issues
Chlorophylls More labile than carotenoids ,
but phaephitins are persistent in sedimentary
records Carotenoids Stability depends on
structure (decreases with the increase of the
number of functional gruoups).
43
Carotenoids
Pigmento Grupos Funcionais Afinidade taxonômica
b,b-caroteno 0 Cianobactérias, algas eucarióticas e plantas vasculares
b,e-caroteno 0 Criptofitas
Aloxantina 2 Cryptofitas
Luteina 2 Clorófitas
Neoxantina 4 Clorófitas
Violaxantina 4 Chrisofitas e Clorófitas
Fucoxantina 5 Chrisofitas e Diatomáceas
Diatoxantina 2 Diatomáceas
Diadinoxantina 3 Dinoflagelados, Crisofitas e Diatomáceas
Peridinina 6 Dinoflagelados
Dinoxantina 4 Dinoflagelados
Zeaxantina 2 Cianobactérias, Clorófitas
Myxoxantofila 3 Cianobactérias
Echinenona 1 Cianobactérias e zooplâncton (Cladocera)
Cantaxantina 2 Cianobactérias e zooplâncton (Cladocera)
Astaxantina 4 Zooplâncton (Crustacea)
Okenona 2 Bactérias fotossintéticas (Chromatiaceae)
Scytonemina-1, -2 4 Organismos fotossintéticos expostos a alta radiação UV
(adaptado de Buchaca Catalan 2008)
Estáveis, abundantes
44
Chlorophylls
Pigmento Afinidades taxonômicas
Bacteriofeofitina-a Bactérias fotossintéticas (Rodospirillaceae e Chromatiaceae)
Bacterioclorofila-e Bactérias fotossintéticas (variedades marrons de Chlorobiaceae)
Clorofila-a Razão molar Cl-a/forbinas a como indicador de preservação
Chlorofilídeo-a Produto de degradação da Cl-a, abundante em Diatomáceas
Cl-a (alômero) Produto de degradação da Cl-a
Cl-a (epímero) Produto de degradação da Cl-a
Feofitina-a1, -a2 Produto de degradação da Cl-a (senescência)
Feoforbídeo-a1, -a2, Produto de degradação da Cl-a (grazing)
-a3, -a30, -a4
Clorofila-b Clorófitas
Feofitina-b1, -b2 Produto de degradação da Cl-b
Clorofila-c1 Crisofitas e Diatomáceas
Clorofila-c2 Crisofitas, Diatomáceas, Criptofitas e Dinoflagelados
Clorofila-c3 Crisofitas e Diatomáceas
(adaptado de Buchaca Catalan 2008)
45
UV/VIS absorption of pigments
46
Chlorophylls
Phaeophytin a
Chlorophyll a
- Mg
- Mg
- Phytil
- Mg, -COOMe
Phaephorbide a
Pirophaephytin a
Jeffrey et al.1997
47
Polyene chain chromophore
UV7VIS Electronic transitions
Main transition
Vibrational fine structure
48
Calculation of III/II for a caroteneid
II
III
0
0
Vibrational fine structure
49
Molecular structure x spectroscopic properties
Lenght ? ? ? ?
Chromophore (polyene chain)
carotenoid Conjug. db. bonds ? max (hexane) ? max (hexane) ? max (hexane)
phytoene 3 276 286 297
?-carotene 7 378 400 425
lycopene 11 444 470 502
50
Molecular structure x spectroscopic properties
Geometrical cis-trans isomers small
hypsochromic effect Significant hypochromic
effect Reduction of vibrational fine
structure Appearance of a cis-peak ( 142 nm
below the longest maximum of the
all-rans,measurd in hexane Beta-Rings fine
structure much reduced, ?max shorter than in the
acyclic Acetylenic groups replacement of d.bond
to triple bond - 15-20 nm shorter
wavelength Allenic groups Carbonyl groups
Britton, 1995, Carotenoids, 3 vol, Birkhäuser
51
Molecular environment x spectroscopic properties
Solvent Approx. bathochromic shift1
Hexane, light petroleum, ethanol, diethylether, acetonitrile 0
acetone 2-6
chloroform 10-20
dichlorometane 10-20
benzene 18-24
toluene 18-24
pyridine 18-24
Carbon disulphide 18-24
1 displacement of ?max to longer wavelength
52
Identification of pigments by Mass Spectrometry
53
- HPLC method with improved resolution, LCMS
analysis and the automated acquisition of MS/MS
data for pigments - extracts from a sediment (Priest Pot, Cumbria,
UK), - a microbial mat (les Salines de la Trinital,
South Catalonia, Spain) - a culture (C. phaeobacteroides)
- SEPARATION OF A GREAT NUMBER OF PIGMENTS,
INCLUDING NOVEL BACTERIOCHLOROPHYLL DERIVATIVES.
Airs, 2001
54
More than 60 pigments during the run
Airs, 2001
55
(No Transcript)
56
HPLC coupled both to UV photodiode array
detection and to atmospheric pressure mass
spectrometric techniques (HPLCDAD-APIMS)
Pigments ( chlorophylls, carotenoid),
galactolipids, alkaloids, sterols and
mycosporine-like amino acids,
Frassanito 2005
57
(No Transcript)
58
Extraction and separation of pigments
59
- Chemotaxonomic estimation of phytoplankton
communities in aquatic and sedimentary
environments involves not only the choice of
marker pigments, but also efficient extraction
and separation procedures and a reasonable
treatment of the data obtained. - Extraction must be quantitative for all pigments
- HPLC separation must be able to
- separate simultaneously groups of molecules of
very different polarities - Resolve very similar compounds, for instance
isomers
60
Extraction of phytoplankton pigments
Acetone 90 Acetone 100 Methanol Acetone
Methanol ( 11) N,N-dimetilformamide
(DMF) Buffered Methanol ( 2 NH4Ac 0,5 M)
Solvents
Procedure
Sonication or criogenic homogenization overnight
or immediate extraction
61
Separation (HPLC)
Filtration GF/F 47mm
Extration Methanol NH4Ac 0,5M (982)
Sonification, ice-bath (30 s) Centrifugation
(5 min, 4800 rpm)
62
Chromatographic separation of Phytoplankton
pigments
63
Separation with C30 columns Development of a
computer-assisted method (Software Dry Lab)
Fase estacionária C30 (YMC, C30, 5µm,
polimérica 250x4,6 mm ID Fase móvel
ACH3OHTBA (28 mM) 7030 (v/v) B CH3CH2OH pH
6,5 Gradiente chlorophylls Fig A30-100 B,
50 min Vazão 1,2 ml/min T 47 oC Carotenóides Fi
g B25-63 B, 35 min, 63-100B/13 min Vazão
1,4 ml/min T oC
c3
DV, MV cl b
alo-, diato-xanthins e luteína
c2
DV, MV cl a
c1
luteína
aloxanthin
diatoxanthin
Resolution otimization for chlorophylls And
for carotenoids in Sparate runs
Mistura-teste Van Heukelem e Thomas, Journal of
Chromatography A, 910 (2001) 31-49
Resolution separation mono/divynil clh a, b They
dont separate in C18 !! (depends on aliphatic
chain?)
64
Separation with C8 columns
1) Development of a computer-assisted method
(Software Dry Lab)
DV, MV cl b
Zeaxanthin, luteína,
DV, MV cl a
c3
Fase estacionária C8 (Eclipse XDB, 3,5
µm 150x4,6 mm ID Fase móvel ACH3OHTBAA (28
mM) 7030 (v/v), pH 6,5 B CH3OH
c1 clorofilídeo a
C2 MgDVP
Mistura-teste.Van Heukelem e Thomas, Journal of
Chromatography A, 910 (2001) 31-49
2) Zapata et al., 2000 Mar. Ecol Progr. Ser. 195
29-45, 2000
Fase móvel A CH3OH CH3CN pirid.acet.
(502525) B CH3OH CH3CN acetona (206020)
65
C8, Zapata
R0,8
cl b/DV cl b Rlt 0,5
Zeaxanthin, dihidroluteína
Clor c2
Rgt1
4k Hex /9cis Neo Rgt 1,25
MgDVP
Rlt 0,5
cl b/DV cl b R 0,8
R1
C8, Van Heukelem
4k Hex /9cis Neo Não resolve
Pigment mixture, S. Wright, Course Notes
C8 better for chlorophyll c family
66
Comparison of method sensitivity with C18 and C8
columns
Fases estacionárias C8 (Symmetry C8, 3,5 µm
150 x 4,6 mm) C18 (Supelcosil L-C18, 5 µM 250 x
4,6 mm) Fase móvel Coluna C18 adap. Kraay,
1992 ACH3OHH2O (8515) B CH3CN.H2O (9010) C
Acet. Etila (vazão 0,6 ml/min) Coluna C8
Zapata, 2000
Mendes et al., Limnol. Oceanogr. Methods 5, 2007,
363-370
C18 More sensitivity Lower limit of
detection Better for low concentration pigments
67
Separation of complex samples, method compatible
with LC/MS
Método SCOR 1997
Fase estacionária 2 colunas in line Waters
Spherisorb ODS2 3 µM 150 x 4,6 mm) Fase móvel
A NH4Ac 0,01M B CH3OH C CH3CN D Acet.
Etila Gradiente5A, 85 B, 15 C isocr.5
min, 0A, 20 B,15C,65 D, 95 min, 0A, 1B,
1C, 98D, 5 min,isocr. 5 min Adequado para
LC/MS
Método Airs et Al.
Extrato de amostra de sedimento (Priest Pot)
Airs et al. Journal of Chromatography a 917
(2001) 167-177
68
zeaxanthin
19,-butanoilfuco
luteina
Cl a DV cla
Cl b DV clb
peridinina
diatoxanthin
diadinoxanthin
Cl c2
aloxanthin
neoxanthin
dinoxanthin
violaxanthin
fucoxanthin
prasinoxanthin
Cl c3
- Fase estacionária
- Spherisorb ODS1/ C18
- 250 x 4,6 mm 5 ?m
- Fase móvel
- A CH3OH 0,3 M em
- NH4Ac ACN H20
- (513613)
- B AcetEtila ACN (7030)
- Vazão 1,2 ml/min
- Gradiente0 a 25 B em
- 5 min, isocr.5 min,
- 25 a 100 B
- em 20 min.
Labor. UFF, Cromatógrafo Bischoffanalysentechn.,
Mistura-teste (DHI), 100µL injetados na fase A,
Separates?,?-carotene, ?,?-carotene,
Aloxanthin,Lutein, Neoxanthin, Violaxanthin,
Fucoxanthin, Diatoxanthin,Diadinoxanthin,
Peridinina, Dinoxanthin, Zeaxanthin,
Mixoxantophyll, Equinenone, Cantaxanthin, Astaxant
hin, Okenone, Scytonemin-1, -2,
Bacteriophaeophytin-a, Bacteriochlorophyll-e,
chlorophyll-a, Chlorophilide-a, Chl-a Allomer and
Epimer, phaeophytin- a1, a2, phaeophorbide -a1,
-a2, -a3, -a3, -a4, chlorophyll b, phaeophytin
-b1, -b2, chlorophyll c1, -c2, -c3
Buchaca e Catalan (2008)
69
HOW TO DETERMINE PHYTOPLANKTON ?
ESTIMATION OF THE ABUNDANCE OF PHYTOPLANKTONIC
COMMUNITY BY PIGMENT MARKERS
Based on the contribution, in terms of
chlorophyll a, of each group of taxonomical
class (Chl a)c to total chlorophyll a in the
sample (Chl a)t (Chl a)t (Chl a)c1 (Chl
a)c2 (Chl a)c3 ...... (Chl a)cn
Calculation of (Chl a)cn ?
Easy !
70
METHOD 1
Calculation of (Chl a)c by the choice of one
marker pigment for each class
Class Marker pigment (Pm) Pm/Cla ratio in the class
Cianobactérias zeaxanthin Rzea/cla
Clorophyta luteína Rlut/cla
Dinophyta peridinina Rper/cla
Cryptophyta aloxanthin Ralo/cla
.......................... ....................... .......................
Bacyllariophyta fucoxanthin Rfuco/cla
Problem Fixed R not necessarily Corresponds To
the ratios In the samples
(Chl a)t Rzea/cla x (Zea) Rlut/cla x
(Lut) ......... Rfuco x (Fuco)
sample
(Chl a)c and of each class
Fixed
71
METHOD 2
Multilinear regression
Sample 1 (Chl a)t1 Rzea/cla x (Zea)1
Rlut/cla x (Lut)1 ......... Rfuco x
(Fuco)1 Sample 2 (Chl a)t2 Rzea/cla x
(Zea)2 Rlut/cla x (Lut)2 ......... Rfuco
x (Fuco)2 ........................................
..................................................
.......................................... Sample
n (Chl a)tn Rzea/cla x (Zea)n
Rlut/cla x (Lut)n ......... Rfuco x (Fuco)n
Unknown Rs, determined by pela resolution of a
system of n equations and n unknowns
?
Rs are determined, but many classes dont have a
specific pigment
(Chl a)cn of ech class
72
MÉTODO 3 Determinação da composição
fitoplanctônica por análise fatorial (MACKEY et
al., 1996)
- Software CHEMTAX problema de análise fatorial
- matriz de dados S concentrações encontradas para
os pigmentos - no ambiente num conjunto de amostras
- fatorizada em matrizes
- F matriz das razões dos pigmentos para as
diferentes classes - de algas puras e
- C abundâncias de cada classe de alga em cada
amostra
73
MATRIZ F Razões Ri lpigmi/chlorophyll a para
cada classe
PER BUT FUC HEX NEO PRA VI0L ALO LUT ZEA CLB CLA
Prasinophyta 0 0 0 0 0,061 0,127 0 0,004 0 0 0,381 0,403
Dinophyta 0,515 0 0 0 0 0 0 0 0 0 0 0,485
Cryptophyta 0 0 0 0 0 0 0 0,186 0 0 0 0,814
Haptophyta3 0 0 0 0,630 0 0 0 0 0 0 0 0,370
Haptophyta4 0 0,104 0,247 0,227 0 0 0 0 0 0 0 0,422
Chorophyta 0 0 0 0 0,040 0 0,035 0 0,127 0,006 0,165 0,628
Synecho. 0 0 0 0 0 0 0 0 0 0,258 0 0,742
Diatomaceas 0 0 0,430 0 0 0 00 0 0 0 0,570
C contribuição de cada classe (a ser
determinada)
MATRIZ S experimental
Amostra 1 (Chl a)t1 (Zea)1 (Lut)1
....... (Fuco)1 Amostra 2 (Chl a)t2
(Zea)2 (Lut)2 ....... (Fuco)2 ..............
.... ............ .........
........ ....... Amostra n (Chl a)tn
(Zea)n (Lut) ....... (Fuco)n
Clpras ClDin ClCryp ClHapt3 ClHapt4 ClChlor ClSyn
ClDiatom
F x C S
74
Para uma fatorização de S que tenha um
significado físico F variável, Fo dados da
literatura (normalizados/Cl a) Estimativa
inicial da matriz de abundâncias das classes
(Co) calculada resolvendo-se a equação de
mínimos quadrados
minimizar ?? S Co Fo ??, sob as
condições Coij ? 0 ? i, j ? Coij 1
? j
O resíduo é expresso por
?o ?? S Co Fo ??
Um algoritmo de decréscimo máximo do resíduo
foi usado (variação dos elementos de F, 10 a
cada iteração)
75
Juturnaíba reservoir as a study model
Marcelo Marinho e Silvana V. Rodrigues
76
OBJETIVOS
- Avaliar a aplicabilidade do método de análise de
pigmentos por HPLC - para detecção das variações na biomassa e
composição do fitoplâncton, comparando com os
dados obtidos por microscopia
77
METODOLOGIA
- Fitoplâncton
- Coletas quinzenais - jun/96 - mai/97 (estação
central) - Biovolume
- método de sedimentação (Utermöhl, 1958)
- Pigmentos
Amostra (0,25 - 1,8 L)
Injeção e análise HPLC
CONDIÇÕES CROMATOGRÁFICAS
- Filtração (GF/C)
- Congelamento
- (CO2 sólido)
- Coluna C18 - fase reversa
- Gradiente alta pressão
- (modificado de Garrido Zapata, 1993)
- Detecção - 440nm
Extração Metanol 100
78
Biomass (chlorophyll a)
Contribution calculated by marker pigments
Razão Xan/Chl-a
CHEMTAX
79
Biovolume 0,2 L Lugols solution sedimentation
method (Utermöhl, 1958) biomass product of
population and mean unit volume of each
species (specific density of cells 1 g/cm3,
cell size mean of at least 30 measurements)
80
Biomass (Biovolume)
81
(No Transcript)
82
Biomass (CHEMTAX) x Biomass (biovolume)
2 periods in both methods
CHEMTAX Period 1 (June - November 96) 3.7
- 36.4 mg/L chl a Chlorophyceae, Cyanobacteria,
Cryptophyceae Period 2 (December 96- May 97)
46.9 - 254.4 mg/L chl a 81 to 99
Cyanobacteria.
83
CONCLUSIONS
- High correlation between biovolume and Chl-a.
Chl-a can be used as a parameter to estimate
biovolume. - Interpretation of pigment data with CHEMTAX
better correlation with biovolume than that based
on Xan/Chl-a ratios from unialgal cultures. - Only Chlorophyceae and Dinophyceae did not
present significant correlation with cell count. - Similar general pattern of the phytoplankton
community dynamics by cell count and pigment
analysis two periods and the Cyanobacteria bloom
recorded.
84
GUANABARA BAY RJ/BRAZIL
12 SAMPLING SITES SAMPLING FREQUENCE - 12
CAMPAIGNS - JANUARY TO AUGUST (SUMMER/AUTUMN)
2006
85
HOMOGENEITY OF SAMPLES WITHIN EACH DATA MATRIX
Data processing CHEMTAX Samples divided in
5 environmentally different groups
5
2
3
4
1
86
(No Transcript)
87
(No Transcript)