Diapositive 1 - PowerPoint PPT Presentation
1 / 29
Title:
Diapositive 1
Description:
... / UCLA Faculty Research Grant NSF CAREER Award / Thieme Chemistry Journal Award / Dupont Young Professor Award Boehringer Ingelheim New Investigator Award ... – PowerPoint PPT presentation
Number of Views:103
Avg rating:3.0/5.0
Title: Diapositive 1
1
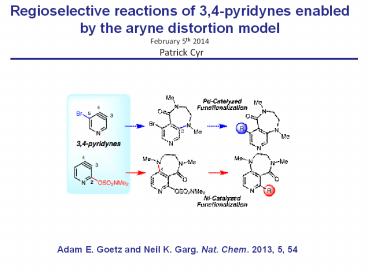
Regioselective reactions of 3,4-pyridynes
enabled by the aryne distortion model February
5th 2014 Patrick Cyr
Adam E. Goetz and Neil K. Garg. Nat. Chem. 2013,
5, 54
2
Neil Garg
- 2000 - B. S. Chem. at New York University
- 2000-2005 - Ph.D. at Caltech (Pr. Brian Stoltz)
- 2005-2007 - N.I.H. postdoctoral fellow at
University of California (Ir. - Pr. Larry
Overman) - 2007 - Assistant Professor at U.C.L.A .
- 2012 - Associate Professor at U.C.L.A.
- 2013 - Professor at U.C.L.A.
- Awards UCLA Faculty Career Development Award /
UCLA Faculty Research Grant NSF CAREER Award /
Thieme Chemistry Journal Award / Dupont Young
Professor Award Boehringer Ingelheim New
Investigator Award . - 41 publications in 7 years
3
Gargs research interest
4
Aryne chemistry
Stoermer Kahlert
1902
- First (possible) suggestion of Aryne
5
Aryne chemistry
Wittig
1942
- First suggestion of Benzyne
- Switterionic intermediate
6
Aryne chemistry
Gilman
1945
- First strong indication for a Benzyne
intermediate
7
Aryne chemistry
Roberts
1953
- 14C labeling experiment proof of a symmetrical
intermediate
8
Aryne chemistry
Wittig and Levine
1955
- First trapping of the benzyne intermediate
- First postulation of Pyridyne
9
Aryne chemistry
Kauffmann
1962
- First trapping of the pyridyne intermediate
10
Characteristic feature of Arynes
- Electrophilic specie
- Biradical character
- Gampe, C. M. Carreira, E. M. Angew. Chem. Int.
Ed. 2012, 51, 3766. - Wenk, H. H. Winkler, M. Sander, W. Angew.
Chem. Int. Ed. 2003, 42, 502.
11
Benzyne preparation
Kobayashi, Chem. Lett.1983, 1211.
Tadross, P. M. Stoltz, B. M. Chem. Rev. 2012,
112, 3550.
12
Aryne distortion model
P character
- Guitiàn. Synlett 1998, 157.
- Angew. Chem. Int. Ed. 2010, 49, 9132-9135.
- J. Am. Chem. Soc. 2010, 132, 1267-1269.
13
Aryne distortion model
Prediction trick
- Addition places negative charge close to
electron withdrawing group
- Guitiàn. Synlett 1998, 157.
- Angew. Chem. Int. Ed. 2010, 49, 9132-9135.
- J. Am. Chem. Soc. 2010, 132, 1267-1269.
14
Hetarynes regioselectivity prediction
- Goetz, A. E. Bronner, S. M. Cisneros, J. D.
Melamed, J. M. Paton, R. S. Houk, K. N.
Garg, N. K. Angew. Chem. Int. Ed. 2012, 51, 2758
2762
15
Previous regioselective inductions
3,4 pyridyne from 3-halo
- Mal, D. Senapati, B. K. Pahari, P. Synlett
2005, No. 6, 994. - Vinter-Pasquier, K. Jamart-Grégoire, B.
Caubère, P. Heterocycles 1997, 45, 2113.
16
Previous regioselective inductions
3,4 pyridyne from Kobayashis precursor
- Tsukazaki, M. Snieckus, V. Heterocycles 1992,
33, 533. - Diaz,M. T. Cobas, A. Guitian, E. Castedo, L.
Synlett 1998, 157.
17
Gargs predicted Effect of substituents
- DFT calculations
18
Regioselectivity studies
19
Regioselectivity studies
20
Regioselectivity studies
21
Regioselectivity studies
22
Derivatizations of pyridyne adducts
Reductive cleavage
- First example of a reductive sulfamate cleavage
23
Derivatizations of pyridyne adducts
Using synthetic handles
24
Conclusion
- First general method to control the 3,4-pyridyne
regioselectivity - Versatile synthetic handles were used as
directing groups - Improvement of the post-transformation of the
sulfamate moiety needed - Further validation of the aryne distortion model
as a predictive tools for the - regioselectivity on hetaryne
- This work should promote the use of pyridyne in
total synthesis and - in pharmaceutical industries
25
Back-up
Benzyne
Tokiwa, H. Akai, s. et al. J. Org. Chem. 2013,
78, 2965.
26
Back-up
precursor synthesis
27
Back-up
Comparison between a C5 Bromide and Chloride
28
Back-up
predicted Effect of substituents
29
Back-up
3,4 -pyridyne
Tsukazaki, M. Snieckus, V. Heterocycles 1992,
33, 533.
Vinter-Pasquier, K. Jamart-Grégoire, B.
Caubère, P. Heterocycles 1997, 45, 2113.
Diaz,M. T. Cobas, A. Guitian, E. Castedo, L.
Synlett 1998, 157.