Determining Limiting Reagents Guided Practice Problem - PowerPoint PPT Presentation
Title:
Determining Limiting Reagents Guided Practice Problem
Description:
Determining Limiting Reagents Guided Practice Problem Part of the SO2 that is introduced into the atmosphere ends up being converted to sulfuric acid, H2SO4. – PowerPoint PPT presentation
Number of Views:138
Avg rating:3.0/5.0
Title: Determining Limiting Reagents Guided Practice Problem
1
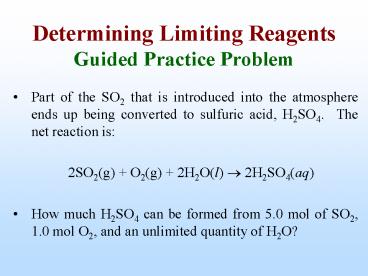
Determining Limiting Reagents Guided Practice
Problem
- Part of the SO2 that is introduced into the
atmosphere ends up being converted to sulfuric
acid, H2SO4. The net reaction is - 2SO2(g) O2(g) 2H2O(l) ? 2H2SO4(aq)
- How much H2SO4 can be formed from 5.0 mol of SO2,
1.0 mol O2, and an unlimited quantity of H2O?
2
Determining Limiting Reagents Guided Practice
Problem
- Consider the following reaction
- 2Na3PO4(aq) 3Ba(NO3)2(aq) ? Ba3(PO4)2(s)
6NaNO3(aq) - Suppose that a solution containing 3.50 g of
Na3PO4 is mixed with a solution containing 6.40 g
of Ba(NO3)2. How many grams of Ba3(PO4)2 can be
formed? What is the yield, if experimentally,
only 4.70 g were obtained from the reaction?
3
Chapter 4Aqueous Reactions and Solution
Stoichiometry
CHEMISTRY The Central Science 9th Edition
4
Solution Composition
- Solutions are homogenous mixtures of two or more
substances - Solute present in smallest amount and is the
substance dissolved in the solvent. - Solvent present in the greater quantities and is
used to dissolve the solute. - Example NaCl dissolved in Water (water Solvent
and NaCl Solute) - Change concentration by using different amounts
of solute and solvent. - Molarity Moles of solute per liter of solution.
5
Concentrations of Solutions
- Formula for Molarity
- The most widely used way of quantifying
concentration of solutions in chemistry.
Molarity is generally represented by the symbol M
and defined as the number of moles of solute
dissolved in a liter of solution.
6
Class Guided Practice Problem
- Calculate the molarity of a solution made by
dissolving 23.4 g of sodium sulfate, Na2SO4, in
enough water to form 125 mL of solution.
Class Practice Problem
- Calculate the molarity of a solution made by
dissolving 5.00 g of NaCl in sufficient water to
form 0.125 L of solution.
7
Class Guided Practice Problem
Determining Mass using Molarity
- Key concept If we know molarity and liters of
solution, we can calculate moles (and mass) of
solute.
- How many grams of C6H12O6 are required to make
100 mL of 0.278 M C6H12O6?
Class Practice Problem
- How many grams of NaCl are required to make a 1 L
of 0.500 M NaCl?
8
Concentration of Diluted Solutions
- Solutions are routinely prepared in stock
solutions form. - Example 12 M HCl
- Solution of lower concentrations are prepared by
adding more solvent (e.g., water), a process
called dilution. - We recognize that the number of moles are the
same in dilute and concentrated solutions. - Hence, moles solute before dilution moles
solute after dilution - So
- MdiluteVdilute MconcentratedVconcentrated
- or
- Mfinal Vfinal MinitialVinitial
9
Class Guided Practice Problem
- How much 3.0 M H2SO4 would be required to make
500 mL of 0.10 M H2SO4? - How many milliliters of 5.0 M K2Cr2O7 solution
must be diluted in order to prepare 250 mL of
0.10 M solution?
Class Practice Problem
10
General Properties of Aqueous Solutions
- Electrolytic Properties
- Aqueous solutions, solutions in water, have the
potential to conduct electricity. - The ability of the solution to conduct depends on
the number of ions in solution. - There are three types of solution
- Strong electrolytes,
- Weak electrolytes, and
- Nonelectrolytes.
11
General Properties of Aqueous Solutions
Electrolytic Properties
12
General Properties of Aqueous Solutions
- Molecular Compounds in Water
- Molecular compounds in water (e.g., CH3OH) no
ions are formed. - If there are no ions in solution, there is
nothing to transport electric charge.
13
General Properties of Aqueous Solutions
- Ionic Compounds in Water
- Ions dissociate in water (NaCl).
- In solution, each ion is surrounded by water
molecules. - Transport of ions through solution causes flow of
current. - Other substances that are not ionic compound
dissociate in water to form ions. - For example (HCl)
14
General Properties of Aqueous Solutions
- Strong and Weak Electrolytes
- Strong electrolytes completely dissociate in
solution. - For example
- Weak electrolytes produce a small concentration
of ions when they dissolve. - These ions exist in equilibrium with the
unionized substance. - For example
15
General Properties of Aqueous Solutions
- Some General Terms
- Acids - substances that able to ionize in
solution to form hydrogen ion (H) and increase
the concentration of H in the solution. - For example, HCl dissociate in water to form H
and Cl- ions. - Bases - are substances that can react with or
accept H ions. - For example, OH- will accept H from HCl forming
H2O. - Salts - are ionic compounds that can be formed by
replacing one or more of the hydrogen ions of an
acid by a different positive ion. - For example, NaCl instead of HCl.
16
General Properties of Aqueous Solutions
- Identifying Strong and Weak Electrolytes
- Most salts are strong electrolytes (NaCl, CaCO3.
- Most acids are weak electrolytes. However, HCl,
HBr, HI, HNO3, H2SO4, HClO3, and HClO4 are strong
acids. - The common strong bases are the hydroxides,
Ca(OH)2, of the alkali metals and the heavy
alkaline earth metals. - Most other substances are nonelectrolytes.
17
Precipitation Reactions
- Exchange (Metathesis) Reactions
- When two solutions are mixed and a solid is
formed, the solid is called a precipitate. - Metathesis reactions involve swapping ions in
solution - AX BY ? AY BX.
- HCl NaOH ? NaCl H2O
- Metathesis reactions will lead to a change in
solution if one of three things occurs - an insoluble solid is formed (precipitate),
- formation of either a soluble weak or
nonelectrolytes, - an insoluble gas is formed.
18
Class Practice Problem
- Write a balanced equation for the reaction
between phosphoric acid, H3PO4, and potassium
hydroxide, KOH.
- H3PO4 3KOH ? 3H2O K3PO4
- AX BY ? AY BX
19
Precipitation Reactions
- Writing Reaction Equations
- Ionic equation used to highlight reaction
between ions. - Molecular equation all species listed as
molecules - HCl(aq) NaOH(aq) ? H2O(l) NaCl(aq)
- Complete ionic equation lists all ions
- H(aq) Cl-(aq) Na(aq) OH-(aq) ? H2O(l)
Na(aq) Cl-(aq) - Net ionic equation lists only unique ions
- H(aq) OH-(aq) ? H2O(l)
20
Class Guided Practice Problem
- Write the net ionic equation for the reactions
that occur when solutions of KOH and Co(NO3)2 are
mixed.
- 2OH- Co2 ? Co(OH)2
21
Acid-Base Reactions
- Acids with one acidic proton are called
monoprotic (e.g., HCl). - Acids with two acidic protons are called diprotic
(e.g., H2SO4). - Acids with many acidic protons are called
polyprotic.
22
Acid-Base Reactions
- Identifying Strong and Weak Electrolytes
- Water soluble and ionic strong electrolyte
(probably). - Water soluble and not ionic, but is a strong acid
(or base) strong electrolyte. - Water soluble and not ionic, and is a weak acid
or base weak electrolyte. - Otherwise, the compound is probably a
nonelectrolyte.
23
Acid-Base Reactions
Strong and Weak Electrolyte Summary
24
Acid-Base Reactions
- Neutralization Reactions and Salts
- Neutralization occurs when a solution of an acid
and a base are mixed - HCl(aq) NaOH(aq) ? H2O(l) NaCl(aq)
- Notice we form a salt (NaCl) and water.
- Salt ionic compound whose cation comes from a
base and anion from an acid. - Neutralization between acid and metal hydroxide
produces water and a salt.
25
Acid-Base Reactions
- Acid-Base Reactions with Gas Formation
- Sulfide and carbonate ions can react with H in a
similar way to OH-. - 2HCl(aq) Na2S(aq) ? H2S(g) 2NaCl(aq)
- 2H(aq) S2-(aq) ? H2S(g)
- HCl(aq) NaHCO3(aq) ? NaCl(aq) H2O(l) CO2(g)
26
Oxidation-Reduction Reactions
- Oxidation and Reduction
- When a metal undergoes corrosion it loses
electrons to form cations - Ca(s) 2H(aq) ? Ca2(aq) H2(g)
- Oxidized atom, molecule, or ion becomes more
positively charged. - Oxidation is the loss of electrons.
- Reduced atom, molecule, or ion becomes less
positively charged. - Reduction is the gain of electrons.
27
Oxidation-Reduction Reactions
Oxidation and Reduction
28
Oxidation-Reduction Reactions
- Oxidation Numbers
- Oxidation number for an ion the charge on the
ion. - Oxidation number for an atom the hypothetical
charge that atom would have if it was an ion. - Oxidation numbers are assigned by a series of
rules - If the atom is in its elemental form, the
oxidation number is zero. E.g., Cl2, H2, P4. - For a monoatomic ion, the charge on the ion is
the oxidation state.
29
Oxidation-Reduction Reactions
- Oxidation Numbers
- Nonmetal usually have negative oxidation numbers
- Oxidation number of O is usually 2. The
peroxide ion, O22-, has oxygen with an oxidation
number of 1. - Oxidation number of H is 1 when bonded to
nonmetals and 1 when bonded to metals. - The oxidation number of F is 1.
- The sum of the oxidation numbers for the atom is
the charge on the molecule (zero for a neutral
molecule).
30
Oxidation-Reduction Reactions
- Oxidation of Metals by Acids and Salts
- Metals are oxidized by acids to form salts
- Mg(s) 2HCl(aq) ? MgCl2(aq) H2(g)
- During the reaction, 2H(aq) is reduced to H2(g).
- Metals can also be oxidized by other salts
- Fe(s) Ni2(aq) ? Fe2(aq) Ni(s)
- Notice that the Fe is oxidized to Fe2 and the
Ni2 is reduced to Ni.
31
Oxidation-Reduction Reactions
- Activity Series
- Some metals are easily oxidized whereas others
are not. - Activity series a list of metals arranged in
decreasing ease of oxidation. - The higher the metal on the activity series, the
more active that metal. - Any metal can be oxidized by the ions of elements
below it.
32
(No Transcript)
33
Solution Stoichiometry and Chemical Analysis
- There are two different types of units
- laboratory units (macroscopic units measure in
lab) - chemical units (microscopic units relate to
moles). - Always convert the laboratory units into chemical
units first. - Grams are converted to moles using molar mass.
- Volume or molarity are converted into moles using
M mol/L. - Use the stoichiometric coefficients to move
between reactants and product.
34
Solution Stoichiometry and Chemical Analysis
35
Solution Stoichiometry and Chemical Analysis
- Titrations
36
Solution Stoichiometry and Chemical Analysis
- Titrations
- Suppose we know the molarity of a NaOH solution
and we want to find the molarity of an HCl
solution. - We know
- molarity of NaOH, volume of HCl.
- What do we want?
- Molarity of HCl.
- What do we do?
- Take a known volume of the HCl solution, measure
the mL of NaOH required to react completely with
the HCl.
37
Solution Stoichiometry and Chemical Analysis
- Titrations
- What do we get?
- Volume of NaOH. We know molarity of the NaOH,
we can calculate moles of NaOH. - Next step?
- We also know HCl NaOH ? NaCl H2O. Therefore,
we know moles of HCl. - Can we finish?
- Knowing mol(HCl) and volume of HCl (20.0 mL
above), we can calculate the molarity.