The Chemistry of Fire - PowerPoint PPT Presentation
1 / 70
Title:
The Chemistry of Fire
Description:
... Rhode Island, United States; it is considered to be the fourth deadliest nightclub fire in American ... esp if visibility is limited due to smoke Type ABC ... – PowerPoint PPT presentation
Number of Views:247
Avg rating:3.0/5.0
Title: The Chemistry of Fire
1
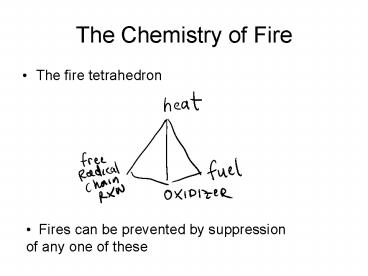
The Chemistry of Fire
- The fire tetrahedron
- Fires can be prevented by suppression of any
one of these
2
Application of the Fire Tetrahedron
- Fuel-
- Shut off the natural gas supply
- Oxidizer
- Close the windows, smother the fire with a
blanket - Heat
- Pour water on the flame, use CO2 extinguisher
- Free radical chain reaction-
- Adsorb radicals with chemical suppressants, salts
3
Fuels
- Organic C, O, H and sometimes N
- Wood is 40-50 cellulose and hemicellulose
- (5 and 6 carbon sugars)
4
Wood tends to produce oxygenated combustion
products
5
Turpenes in turpentine
Nowadays, apart from specialised grades mainly
for artists use (balsam turpentine) turpentine
is a by-product of the production of pulp.
Sulphate turpentine is chilled from the gas
emitted when wood chips are pre-heated with steam
before digestion. Pine and spruce have the best
turpentine yields. Turpentine consists of 45-75
a-pinene (1), 5-30 b-pinene (2), 2-40 3-carene
(3), other turpentines such as limonene and
camphene and their oxidation products, such as
alcohols and aldehydes. http//apps.kemi.se/flode
ssok/floden/kemamne_eng/terpentin_eng.htm
Used in moderation, turpentine is an ideal
accelerant for arson
6
Other fuels
- Cotton mostly cellulose
- Hydrocarbons (CH2)n
7
Other fuels II
- Inorganic fuels
- Mg, Al, S, Zn, etc
- Note surface area is important
- Wood dust ignites easily
- Diesel ignites in a spray, but is difficult to
light in a pool - Metals used in pyrotechnic devices are finely
powdered
8
Oxidizing Agents
- Usually oxygen from air
- In medical facilities can be accelerated
- Temperature greatly affects a fires need for
oxygen - _at_25C need 14-16 O2
- _at_900-1100C flashover conditions- (spontaneous
ignition of entire room) nearly 0 oxygen is
needed - This is the effect of the flammable limit, the
upper and lower concentrations of a flammable gas
and air expressed in fuel that can be ignited
at a specific temperature and pressure
9
Types of Fire
- Flaming combustion
- Common open flame fires like gas burners
- Gas to gas reaction, fuel must be in gaseous
state - Liquids and solids dont burn in an open flame
- these must undergo chemical or phase change first
- Oxygen must be above 10
10
Smoldering Combustion
- Glowing combustion occurs without the generation
of flames - It is a solid to gas reaction
- Surface of solid reacts directly with oxidizer
- Often due to a deficiency of oxidizer
- Less than 10 oxygen is needed
11
Partial or incomplete combustion
- Stoichiometric ratios are rarely involved in
combustion - Oxidation reactions often dont go to completion
- This is called incomplete combustion
- C4H10 13/2 O2 ?
12
Types of Fire
- Flaming combustion
- Common open flame fires like gas burners
- Gas to gas reaction, fuel must be in gaseous
state - Liquids and solids dont burn in an open flame
- these must undergo chemical or phase change first
- Oxygen must be above 10
13
Smoldering Combustion
- Glowing combustion occurs without the generation
of flames - It is a solid to gas reaction
- Surface of solid reacts directly with oxidizer
- Often due to a deficiency of oxidizer
- Less than 10 oxygen is needed
14
Flammable limits
- Fuel/air ratio must be correct for combustion to
occur - There is a minimum and maximum level
- Measured at 0 C and 1 ATM
- Gasoline -1.4 - 7.6 in air
- Acetylene- 4-100
- H2 4-75
- for this reason it is practically impossible
for a full or partly full gas tank to explode or
even burn - The danger comes as temperature increases causing
the range of flammable limits to expand - http//www.youtube.com/watch?vNOTWg3Krww0
15
Examples
- Flash point
- http//www.youtube.com/watch?vyE5LdCyN0aEfeature
related - Flammable limit
- http//www.youtube.com/watch?vICsvddmYMr4
- Auto ignition
- http//www.youtube.com/watch?vlFIiTxqolZk
- Backdraft
- http//www.youtube.com/watch?v91R6MLcf-WQ
16
Partial or incomplete combustion
- Stoichiometric ratios are rarely involved in
combustion - Oxidation reactions often dont go to completion
- This is called incomplete combustion and results
in formation of carbon monoxide.
Complete vs incomplet combustion in a gas pilot
light
17
Effect of Fuel on a Fire
- Fires have either excess air or excess fuel
18
Effect of venting
- Venting a fire has important effects
- Gases inside a room may be oxygen starved
- Gases venting to outside may ignite
- Gases venting into an enclosed room will not
spread a fire - Opening doors and windows may cause a smoldering
fire to reignite
19
Heat
- Sufficient heat is required to produce a
transition from solid to liquid to vapor phase
only vapors burn - Additional energy is also required to initiate
the chemical reaction - Once initiated such reactions are exothermic with
a large increase in entropy
20
Initiation of Fire
- The heat required to initiate a fire is a
critical step - Matches?paper?sticks?wood
- Each step is critical and the underlying process
is to get the wood hot enough that it produces
volatile gases that burn.
21
Types of ignition
- Spontaneous ignition
- Chemical or biological processes that create
sufficient heat to ignite the reacting material - Basically heat is produced faster than it can be
dissipated. - Common with vegetable oils, hay
Spontaneous combustion of hay in a barn
22
Auto-ignition
- Ignition of a material in the absence of flame or
spark (non-piloted ignition) - All combustible materials must reach their
autoignition temperature to burn - Thus one could light paper two ways
- Use a match to heat a small section to ignition
- Heat the entire piece of paper in an oven.
23
Flash Point
- There is a temperature above which a fuel will
flash when presented with a flame this is the
flash point - _at_ 10-20 degrees above the flash point there is
sufficient vapor pressure to sustain a flame - The auto-ignition temperature is the temperature
of spontaneous ignition - For kerosene, the flash point is 100F and the
ignition temperature is 410F.
24
Chemical Chain Reactions
- Reactions become self sustaining when sufficient
heat from exothermic reactions radiates back to
cause ignition away from source - The burning process involves pyrolysis, the
breakdown of solids to produce gases and free
radicals
25
Removal of Free Radicals
- Halon fire extinguishers work by shutting down
the propagation of radicals
Bromine and chlorine quickly shut down free
radicals
26
Types of fire extinguishers
- Type A - Water/firehoses
- Cools fire as water converts to steam
- Causes damage and is dangerous in electrical and
metal fires - Type BC - Powder extinguishers
- 80 NaHCO3
- Starve oxygen and cool by release of CO2
- 6-8 meter range
- Type BC - CO2 gas extinguishers
- Leave no residue for expensive cleanup
- Cool fire and remove oxygen
- 1.5 meter range- can be dangerous for large
electrical fires, esp if visibility is limited
due to smoke - Type ABC Ammonium Phosphate
- Releases ammonia which removes oxygen and yields
phosphoric acid which induces char which
releases fewer volatiles - Type ABC Halon
- Eliminates free radicals, displaces oxygen
- Type D fire extinguisher
- A bucket of sand for metal fires
27
Fires are classified according to the material,
which is being burned. The four classes of fires,
with the American and International symbols, are
as follows Class A Ordinary Combustibles
- Cloth, Wood, Paper, Rubber, many plastics.
ExtinguisherPressurized water (it removes
Heat) suitable for use on Class A only. Dry
chemical mono-ammonium phosphate, (it removes
contact between Oxygen and Fuel), rated for Class
A, B, and C fires.Extinguishers suitable for
Class A fires should be identified by a green
triangle containing the letter "A" and the
pictograph shown above.
http//www.fireadesource.com/faqs.html
28
Class B Flammable Liquids - Gasoline, Oil,
Oil-based paint, Cooking Oil
Extinguisher1) Carbon dioxide (it
displaces Oxygen but dissipates quickly the
combustible surface, if hot, may re-ignite).2)
Dry Chemical (it removes Oxygen from the Fuel by
coating the surface inhibiting the release of
combustible vapors) mono ammonium phosphate,
rated for Class A, B, and C fires Sodium
Bicarbonate and Potassium Bicarbonate, for Class
B and C, preferred for cooking oil fires.3)
Halon it interferes with the fire chemical
reaction by quenching free radicals. Production
has been banned (Montreal, 1998) because Halon
has been found to be an ozone-depleting
substance.Extinguishers that are suitable for
Class B fires are identified by a red square
containing the letter B and the pictograph shown
above.
http//www.fireadesource.com/faqs.html
29
Class C Energized electrical equipment,
including appliances, wiring, circuit breakers,
and fuse boxes.
Extinguisher1) Carbon dioxide (it removes
Oxygen but dissipates quickly the combustible
surface, if still hot, may re-ignite).2) Dry
Chemical (it removes Oxygen from the Fuel by
coating the surface and inhibiting the release of
combustible vapors) mono Ammonium Phosphate,
rated for Class A, B, and C fires Sodium
Bicarbonate and Potassium Bicarbonate, for Class
B and C, preferred for cooking oil fires.3)
Halon it interferes with the fire chemical
reaction by quenching free radicals. Production
has been banned (Montreal, 1998) because Halon
has been found to be an ozone-depleting
substance.Extinguishers suitable for Class C
fires are identified by a blue circle containing
the letter C and the pictograph shown above.
http//www.fireadesource.com/faqs.html
30
Class D Combustible metal such as Mg, Na, Li,
powdered Al, etc.
ExtinguisherExtinguishers rated for class
D fires have a label, which list the types of
metal, on which the extinguisher may be used. The
extinguishing medium must not react with the
burning metal. Extinguishers suitable for Class D
fires are identified by a yellow star containing
the letter D.
http//www.fireadesource.com/faqs.html
31
Fire Retardants
- Barrier theory- chemicals form a glassy barrier
on exposure to heat - Thermal theory chemicals change the thermal
property of the wood to dissapate (conduct) or
absorb (heat capacity)heat - sodium silicates,
chemicals with waters of hydration - Noncombustable gas theory chemicals release
nonflamable gases interfering with combustion -
borax (soduim tetraborate decahydrate) releases
large quantities of water following pyrolysis - Free radical trap theories chemicals release
free radical inhibitors at pyrolytic temperatures
interrupting chain propagation halogens attack
free radicals formed - Increased char theories temperature of
pyrolysis is lowered, directing degradation
towards charring instead of burning, lower
volatile gases borax, NH3PO4 - Most fire retardants operate using several of
these mechanisms
(H2O) x10
NH3 H2P04
Levan, Chemistry of Fire Retardancy in The
Chemistry of Solid Wood http//www.fpl.fs.fed.us
/documnts/pdf1984/levan84a.pdf
32
(No Transcript)
33
Heat Transfer
- Three mechanisms
- Conduction, convection, radiation
- Conductive heating
- Takes place within solids
- Rate is dependant on
- Thermal conductivity heat transfer
within a material - Heat capacity heat required
to raise the temp of a substance 1 degree C - Density g/cm3
34
Thermal Conductivity
- Thermal intertia ? density, heat capacity,
thermal conductivity - At equilibrium, density and heat capacity become
unimportant - Thermal conductivity rules
- Pipes and metal fittings produce fire spread and
structural damage - Thus thermal intertia is maintly important in the
early stages of a fire
35
Thermal properties of selected materials
material Thermal conductivity W/mK Density kg/m3 Heat capacity J/kgK
copper 387 8940 380
concrete 0.8 1900 880
pine 0.14 640 2850
polyethylene 0.35 940 1900
NFPA 921-14
36
Convection
- Transfer of heat energy through the movement of
liquids or gases - Heat is then transferred to a cooler solid
- Rate is a ftn of
- Temperature
- Surface area
- Velocity of gases
- Convection is extremely important in the early
stages of a fire - Hot gases rise to upper portions of the room
- Then they mushroom down
- As heat builds, flashover occurs and entire room
ignites - Hot gases then spread fire through the rest of
the building
37
Radiation
- Transfer of heat through infrared energy
- Radiative power s(T)4
- where s 5.67 x 10-8 (watts/m2)/K4
- Stefans law of radiation
- Thus
- Radiative power becomes highly significant at
elevated temperatures
38
Fire development
- There is a sequence of events which begin as a
fire evolves - Incipient stage
- Ceiling layer development
- Preflashover -
- Flashover
- Post flashover
- Not all fires will go through the entire process
39
Incipient phase
- Incipient room doesnt heat
- Can be short time accellerant
- Or long- spontaneous combustion
- Oily rags (linseed oil) dust or even grass
clipping - Build up of heat due to chemical or bacterial
action - For ignition to occur material must be
- In a gaseous state
- At sufficient concentration to form a flammable
air/gas mixture - Exposed to activation energy of
- Match, spark, friction
40
Incipient stage
- Small flames progress upwards and produce hot
gases. - Smoke begins to accumulate
- Average temperature is just above ambient
41
Emergent Smolder
- Fuel vapors must be raised to higher than
ignition temperature - Some solid materials begin to burn by smoldering
- A hazardous situation as incomplete combustion ?
release of CO and other toxins - Smoldering is a pyrolytic process in which
chemical bonds begin to break, gases are released
and free radicals form
42
Growth (open burning)
- Room begins to heat up
- Oxygen concentration still high or unchanged
- Fire burns up and out as it moves across the
ceiling looking for a way up
43
Ceiling layer development
- Also called the growth phase.
- Smoke increases and begins to accumulate at the
ceiling level - Room heats up and other items begin to burn
- Hot smoke creates a negative pressure in the
room. - There is essentially two layers of heat in the
room, a hot upper layer and a cooler rest of the
room.
44
Preflashover
- Smoke and hot gas layer at ceiling reaches
400-500 C - Rate of heat transfer increases
- Burning rate is fuel controlles and sufficient
oxygen is present - Items in the room begin to pyrolyze notice
smoke given off by chair
45
Flashover
- Temperature in the room rises to the point that
all materials spontaneously combust - Flashover can simulate arson fire as multiple
points in the room ignite - Windows break due to thermal stress, Floor to
ceiling charring will occur due to radiative
heating of all exposed surfaces - Freeburning occurs until ventilation is limited
- Fires can selflimit if nearby fuel isnt present
of it initial fire is too small to ignite
adjacent materials
46
Flashover
- Hot gas reached a critical temperature of 600 C
and ignites, significantly increasing the radiant
heat transferred to floor - Whole room is suddenly and completely engulfed in
flame - Transition lasts only a few seconds
47
Full Room Invovement ? Ventilation Control
?Backdraft
- Room transitions to oxygen regulated smoldering
- The point at which the amount of O2 regulates the
fire - Fire itself is slow smoldering producing large
amounts of CO - If a door is opened at this point, hot CO
combines explosively with O2 - Effect can be confused with explosives however
char pattern will occur only at the top of the
room
48
Example Station Nightclub Fire simulation by NIST
http//www.youtube.com/watch?vIxiOXZ55hbc
The Station nightclub fire occurred beginning at
1107 PM EST, on Thursday, February 20, 2003, at
The Station, a glam metal and rock n roll themed
nightclub located at 211 Cowesett Ave in West
Warwick, Rhode Island, United States it is
considered to be the fourth deadliest nightclub
fire in American history, killing 100 people,
four of whom died after being admitted to local
hospitals. The fire was caused when pyrotechnic
sparks, set off by the tour manager of the
evening's headlining band, Great White, ignited
flammable sound insulation foam in the walls and
ceilings around the stage, creating a flash fire
that engulfed the club in 5½ minutes. Some 230
people were injured
At first, there was no panic. Everybody just kind
of turned. Most people still just stood there. In
the other rooms, the smoke hadn't gotten to them,
the flame wasn't that bad, they didn't think
anything of it. Well, I guess once we all started
to turn toward the door, and we got bottle-necked
into the front door, people just kept pushing,
and eventually everyone popped out of the door,
including myself
49
Incipient phase
- Incipient room doesnt heat
- Can be short time accellerant
- Or long- spontaneous combustion
- Oily rags (linseed oil) dust or even grass
clipping - Build up of heat due to chemical or bacterial
action - For ignition to occur material must be
- In a gaseous state
- At sufficient concentration to form a flammable
air/gas mixture - Exposed to activation energy of
- Match, spark, friction
50
Emergent Smolder
- Fuel vapors must be raised to higher than
ignition temperature - Some solid materials begin to burn by smoldering
- A hazardous situation as incomplete combustion ?
release of CO and other toxins - Smoldering is a pyrolytic process in which
chemical bonds begin to break, gases are released
and free radicals form
51
Flashover
- Temperature in the room rises to the point that
all materials spontaneously combust - Flashover can simulate arson fire as multiple
points in the room ignite - Freeburning occurs until ventilation is limited
- Fires can selflimit if nearby fuel isnt present
of it initial fire is too small to ignite
adjacent materials
52
Post flashover
- Also called full room involvement
- Every piece of combustable material in room burns
- Areas under furniture may be relatively spared,
also materials near the influx of oxygen - Examples
http//faberc.org/Images/NIST/Flashoverx3/LivingRo
omFlashover.wmv http//faberc.org/Images/NIST/Fla
shoverx3/ScotchPine.wmv
53
Backdraft
- Oxygen regulated smoldering
- The point at which the amount of O2 regulates the
fire - Fire itself is slow smoldering producing large
amounts of CO - If a door is opened at this point, hot CO
combines explosively with O2 - Windows will blow out
- Effect can be confused with explosives however
char pattern will occur only at the top of the
room
54
(No Transcript)
55
Accelerated vs Nonaccelerated fires
- Originally thought that accelerated fires would
burn hotter. This is not true - Actually modern homes are composed of a lot of
plastic, which basically burns just like
gasoline. (also gasoline burns at the same
temperature as wood) - Only real difference is a faster rate of room
temperature increase. This is due to the faster
rate of heat release with gasoline
56
Effects of Fire/Scene Reconstruction
- Damage to ceiling is 5x that of Floor
- Damage is usually heaviest near origin
- Aligator char is deepest
- Scales are smaller
- Char burn rate 1 in 40 min _at_1400-1600 F
- Glass melts _at_1200F ? becomes running _at_1600F
- Ovid cracks in glass? rapid heating
- Verticle cracks ? slow fire
- Light bulbs above 40W will expand towards origin
due to melting and expansion of gas inside bulb - Burn patterns can help indicate origin
57
Liquid fuel properties
- Melting and boiling points
- Increase with number of carbons within HC class
- Branching and cyclic groups decrease melting and
boiling points (increased disorder) - Double bonds decrease melting and boiling points
- Aromaticity increases melting and boiling points
due to increased polarity - Alcohol groups greatly increase melting and
boiling points
58
BP 254
BP 229 alcohol
BP 183 aromatic
BP 174
BP 181 cyclic
BP 171 alkene
BP160 branched
BP 171
BP 148
59
Specific Gravity
- Compounds lighter than water have lower specific
gravity gm/cc 1 for H2O - Petroleum products generally have a low specific
gravity and float on water (up to asphalt) - General trends-
- Increasing with carbon number for n-alkanes
- Aromatics tend to have higher SG than alkanes
- Compounds with Cl or S tend to have high SG
60
Vapor density
- Volume of vapor or gas compared to air (Air 29
g/mol vapor density of 1) - Air is 78.1 N2, 21 O2, 1 Ar, 0.03 CO2
- This is 21.9g/mol N2 6.7 g/mol O2
- If the vapor density of a gas is below 1 it will
rise. If above 1 if settles at floor level
61
Vapor Density
- Only 14 gases and vapors have a vapor density
less than 1 - acetylene, ammonia, CO, diborane, H2, He,
HCN,HF, CH4, methyl lithium, Ne, N2, H2O - 9 are flammable,
- Many other gases are heavier than air
- Methanol, propane, butane, acetone, pentane,
toluene, etc.
62
Effect of vapor density
Too Rich
Combustion possible
Too lean
Stove
63
Flammability limits
- Lower flammability limit
upper flammability limit - Methane 5.3 14
- Propane 2.2 9.5
- Acetylene 2.5 81
- Butane 1.9 8.5
- Gasoline 1.5 7.6
- Kerosene 1 5
- Diesel 0.5 4.1
- Carbon monoxide 12.5 74
- H 4 75
- HS 4.3 45
- NH3 15.5 45.5
64
Vapor pressure of a liquid mixture
- How to calculate if in an explosion LFL was
reached? - Use Raoults law
- Ptotal Sum(Pn ?n)
- vapor pressure of mixture time sthe molar
fraction of the liquid in the mixture
65
Flash Point calculations
- Flash point is the lowest temperature at which a
substance produces sufficient vapor to form an
ignitable mixture (pilot light) - Gas ignites and then extinguishes. The concept
is important as this is the lowest temperature at
which a risk of fire exists. - Flash points are temperature dependent
- 1000/(Tf273) B0 B1log P25
- Where B0 and B1 are constants (see book) and P25
is the vapor pressure at 25C
66
Summary of concepts
- Melting point
- Boiling point
- Specific gravity
- Vapor density
- Flammability limits lFL, UFL
- Vapor pressure
- Flash point will pop
- Fire point sustains a fire
- Ignition temperature will ignite
- Autoignition temperature will ignite with no
source
67
Thermal Conductivity
- Thermal intertia ? density, heat capacity,
thermal conductivity - At equilibrium, density and heat capacity become
unimportant - Thermal conductivity rules
- Pipes and metal fittings produce fire spread and
structural damage - Thus thermal intertia is maintly important in the
early stages of a fire
68
Thermal properties of selected materials
material Thermal conductivity W/mK Density kg/m3 Heat capacity J/kgK
copper 387 8940 380
concrete 0.8 1900 880
pine 0.14 640 2850
polyethylene 0.35 940 1900
NFPA 921-14
69
Convection
- Transfer of heat energy through the movement of
liquids or gases - Heat is then transferred to a cooler solid
- Rate is a ftn of
- Temperature
- Surface area
- Velocity of gases
- Convection is extremely important in the early
stages of a fire - Hot gases rise to upper portions of the room
- Then they mushroom down
- As heat builds, flashover occurs and entire room
ignites - Hot gases then spread fire through the rest of
the building
70
Radiation
- Transfer of heat through infrared energy
- Radiative power s(T)4
- where s 5.67 x 10-8 (watts/m2)/K4
- Stefans law of radiation
- Thus
- Radiative power becomes highly significant at
elevated temperatures