Classifying Matter - PowerPoint PPT Presentation
Title:
Classifying Matter
Description:
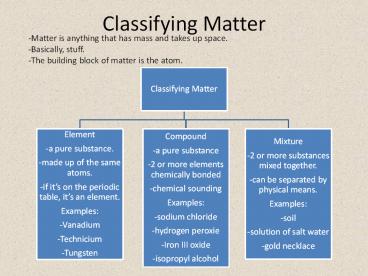
Classifying Matter-Matter is anything that has mass and takes up space. -Basically, stuff.-The building block of matter is the atom. Compounds Ionic Bond: Metal to ... – PowerPoint PPT presentation
Number of Views:238
Avg rating:3.0/5.0
Title: Classifying Matter
1
Classifying Matter
-Matter is anything that has mass and takes up
space. -Basically, stuff. -The building block of
matter is the atom.
2
Compounds
- Ionic Bond
- Metal to nonmetal is always an ionic bond.
- Metals are to the left of the staircase line on
the periodic table (except hydrogen). - The metal will always be first in the formula or
name of the compound. - NaCl
- CuO
- Iron II chloride
- Potassium phosphate
- Covalent Bond
- Nonmetal to nonmetal bond.
- Tend to have numeric prefixes in the name.
- Carbon dioxide
- Water
- PCl3
- N2O4
3
Mixtures
- Homogeneous
- Look the same throughout.
- Solutions are ALWAYS homogeneous mixtures.
- Usually separated by distillation or
chromatography. - Metal alloys tend to be homogeneous mixtures.
- Examples
- Orange juice (no pulp)
- Koolaid
- Gold necklace
- Heterogeneous
- Look different throughout.
- See layers.
- Usually separated by filtration.
- Examples
- Oil vinegar dressing
- Salad
- Soil
- Rocky road ice cream
4
Classify..be specific
- 1. Sodium bicarbonate
- 2. Water
- 3. Soil
- 4. Solution of sugar water
- 5. Phosphorous trichloride
- 6. Carbon Dioxide
- 7. Rocky Road Ice Cream
- 8. Alcohol lol
- 9. Pure Air
- 10. Calcium Gluconate
5
Properties/Changes of Matter
- Physical
- A property that can be observed without changing
the identity of a substance. - Melting point, boiling point, etc.
- Solubility
- Luster, ductility, malleability.
- Color
- Density (mass/volume)
- A change that occurs without changing the
chemical identity of the substance. - Any phase change (boiling, freezing, etc.).
- Dissolving
- Chemical
- A property that can only be observed by changing
the chemical identity of a substance. - Flammability
- Ability to rust
- Ability to tarnish
- A change that occurs and changes the chemical
identity of the substance. - Combustion
- Rusting or Tarnishing
- Rotting or Souring
- Any chemical reaction
- Color change.
- The creation of a new substance in a reaction is
always chemical.
6
Identify Physical of Chemical Change
7
Kinetic Theory of Matter
8
(No Transcript)
9
Phase Change Diagrams