How to study a protein - PowerPoint PPT Presentation
1 / 52
Title: How to study a protein
1
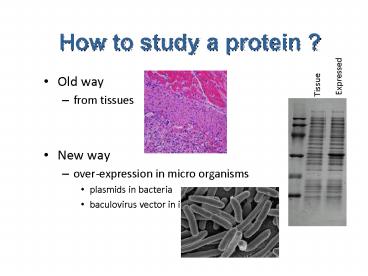
How to study a protein ?
Expressed
Tissue
- Old way
- from tissues
- New way
- over-expression in micro organisms
- plasmids in bacteria
- baculovirus vector in insect cells
2
(No Transcript)
3
Expression of Recombinant Proteins
- Expression Vector Features
- gene dosage
- strong promoter
- inducible promoter
- translation signals, etc
- fusion proteins
- protease defective hosts
4
(No Transcript)
5
Expression vector
6
Preparation of Expression Vector
- subclone insert from current vector to expression
vector - design PCR primers to amplify region of interest
- expressed protein must be
- correct orientation
- in-frame
BamH1 EcoR1 SmaI
SalI XhoI NotI ... ATC GAA GGT CGT GGG
ATC CCC AGG AAT TCC CGG GTC GAC TCG AGC GGC CGC
... ... TAG CTT CCA GCA CCC TAG GGG TCC TTA AGG
GCC CAG CTG AGC TCG CCG GCG ... ... Ile Glu Gly
Arg Gly Ile Pro Arg Asn Ser Arg Val Asp Ser Ser
Gly Arg ... Factor Xa
7
- increase stability
- affinity purification
- detection/assay
- spectrophotometric
- binding assays
- antibodies
- export signals
Fusion Proteins
8
Transfection Methods
- calcium phosphate
- DEAE-dextran
- electroporation
- liposomes
- protoplast fusion
- ballistics (gene gun)
- microinjection
9
- Possible IB Cure
- isolate by differential centrifugation
- solubilize in urea
- re-nature protein (?)
10
Problems with Expression of Eukaryotic Proteins
in Prokaryotes
- stability (protein and gene)
- proper folding and disulfide formation
- post-translational modifications
- asking species specific questions
11
- Post-translational modifications
- Phosphorylation
- Ubiquitination (proteasomal degradation)
- Methylation
- Acetylation
- Glycosylation (N-linked, O-linked)
- Oxidation
- Nitrosylation, etc.
12
Eukaryotic Expression Systems
- in theory, plasmids can be introduced
- into any host
- yeast are easy to maintain in lab
- Saccharomyces cerevisiae
- Pichia pastoris
- viruses
- several mammalian
- baculovirus (insect)
vaccinia (lytic) adenovirus (lytic) papilloma
(episomal) retrovirus (integrated)
13
Protein Purification Techniques Large Scale
- Salting out
- Dialysis
- Gel-Filtration Chromatography
- Ion-Exchange Chromatography
- Affinity Chromatography
- High-Pressure Liquid Chromatography
14
Differential centrifugation as a first step in
protein purification
15
Gel-Filtration Chromatography Separation based
on size
16
17
High Pressure Liquid Chromatography
High pressure limits diffusion and increases
interactions with chromatography media HPLC gives
very high resolution of protein components
18
Ion-Exchange Chromatography Separation based on
charge
19
Types of ion-exchange chromatography media
20
Affinity Chromatography
Small molecules are attached to beads and complex
protein mixtures are applied. Bound proteins
can be eluted with the small molecule or with
denaturing reagents (urea, guanidine, etc.)
21
Elution fractions from affinity chromatography
Fraction number
25
35
pure protein
22
1. Engineered protease site allows removal of
fusion partner
23
2. Addition of a few residues should have minimal
effect on recombinant protein
- His6 Tag
- add 6 consecutive His to either end
- binds metals
- Epitope Tag
- 6-12 amino acids
- mAb for detection or purification
24
Mass spectrometry of Proteins
25
What is a mass spectrum?
26
MS of proteins is used for
- Identification of proteins
- Exact mass of a protein
- Peptide mass fingerprint
- Aminoacid sequence
- Protein characterization
- Posttranslational modifications
- De Novo sequencing
- Analysis of complex protein mixtures
- Quantification of compounds
27
How does it work?
- Sample preparation
- Isolation, purification, de-salting
- Digestion with selective proteolytic enzyme
- Ionisation
- MALDI (Matrix Assisted Laser Desorption
Ionisation) - Electrospray
- Ion detection and mass measurment
28
Samples
- Protein solutions, protein mixtures,
- bands from SDS PAGE, 2D Gel spots
- Whole protein
- Protein digest
29
Trypsin digestion
- Trypsin is a selective proteolytic enzyme
- Cuts after a Lys (K) and after Arg (R) aminoacid
residue
Denaturation (Heat, SDS)
Trypsin digestion 12h _at_ 37C
Protein
Peptides
MKLLILTCLVAVALARPKHPIKHQGLPQEVLNENLLRFFVAPFPEVFGKE
KVNELSK DIGSESTEDQAMEDIKQMEAESISSSEEIVPNSVEQKHIQKE
DVPSERYLGYLEQLL RLKKYKVPQLEIVPNSAEERLHSMKEGIHAQQKE
PMIGVNQELAYFYPELFRQFYQL DAYPSGAWYYVPLGTQYTDAPSFSDI
PNPIGSENSEKTTMPLW
30
Trypsin digestion
- Trypsin is a selective proteolytic enzyme
- Cuts after a Lys (K) and after Arg (R) aminoacid
residue
Denaturation (Heat, SDS)
Trypsin digestion 12h _at_ 37C
Protein
Peptides
MK LLILTCLVAVALAR PKHPIKHQGLPQEVLNENLLR
FFVAPFPEVFGKEKVNELSKDIGSESTEDQAMEDIK
QMEAESISSSEEIVPNSVEQK HIQK EDVPSER
YLGYLEQLLR LK K YK VPQLEIVPNSAEER LHSMK
EGIHAQQK EPMIGVNQELAYFYPELFR
QFYQLDAYPSGAWYYVPLGTQYTDAPSFSDIPNPIGSENSEK
TTMPLW
31
Ionization
- 2 methods used in protein MS
- MALDI Electrospray
32
MALDI
Matrix Assisted Laser Desorption Ionization
33
MALDI
Matrix Molecules
- Acidic- act as a proton source to encourage
ionization of the analyte - Have a strong optical absorption in the UV, so
that they rapidly and efficiently absorb the
laser irradiation - Give the analyte molecule a single, sometimes
double charge
34
Electrospray
- No matrix necessary
- Analyte in acidic solution
- gains a positive charge (H)
- Positive ions form
- spray in electric field
- Produces multiple
- charged ions
35
Electrospray
36
How a mass spectrum is created
- ToF Time of Flight
- Measures time from ion entry to the flight
chamber to its detection on the detector - Light ions are fast, more massive ions are slower
- A double charged ion is 2x faster
37
Time of Flight
Start Detector
1x 1x 2x
The mixture of ions is separated by their
velocity in electric field
38
Time of Flight
Start Detector
1x 1x 2x
The measured mass spectrum is a ratio of mass to
charge, m/z
39
Mass spectrum
Ion intensity
Mass/charge
40
Protein Identification
41
Peptide Mass Fingerprint
- Trypsin cleaves the protein in defined positions
selective digestion - The resulting peptides
- can be predicted (software)
- are characteristic for a protein
- their masses (m/z) can be found in protein
databases - PMF can be used to identify a known protein
42
MS peptide sequencing
- Fragmentation of a peptide precursor by
collision with atoms of inert gas Argon - Fragmenting occurs on defined sites of the chain
the peptide bond - The mass of the fragment is characteristic for a
specific aminoacid - Partial fragments can be used to identify a
sequence
43
WTF?
44
Collision Induced Dissociation (CID)
- Peptide fragmentation
- precursor product ions
fragmentation
45
Peptide sequencing
- Sequence AVDDFLISLDGTANK
- Database search
- BLASTp
Protein identification
46
Protein identification
- Database search (Swissprot, NCBI, custom db)
- Known proteins, sequenced genomes, translated
cDNA libraries, conserved sequences - De Novo sequencing / BLASTp
- Unknown proteins, sequence homologies
- At least 3 peptides to identify a protein (PMF)
- At least 3 fragments to identify a peptide
47
(No Transcript)
48
MS in combination with chromatographic separation
methods
- Gas chromatography (GC-MS)
- Not suitable for proteins
- Liquid chromatography (LC-MS)
- Wide selection of analytic columns
- C4 - C18 for proteins and peptides
- Hydrophilic Interaction for polar substances
- Allows pre-separation of analyzed mixture
- High sensitivity
- High resolution
49
(No Transcript)
50
(No Transcript)
51
Appendix 1 1x charge vs 2x charge
52
Appendix 2 b ions and y ions