Principles%20of%20Bioinorganic%20Chemistry - PowerPoint PPT Presentation
Title:
Principles%20of%20Bioinorganic%20Chemistry
Description:
You should have your paper topic approved by Prof. Lippard this week, if you ... Found in spiny lobsters, crayfish, and arachnids. Deoxy Hc, colorless, dicopper(I) ... – PowerPoint PPT presentation
Number of Views:202
Avg rating:3.0/5.0
Title: Principles%20of%20Bioinorganic%20Chemistry
1
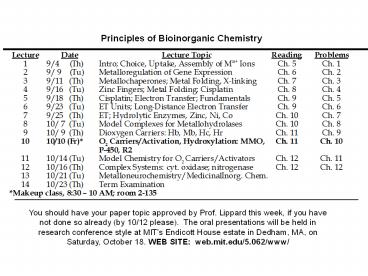
Principles of Bioinorganic Chemistry
You should have your paper topic approved by
Prof. Lippard this week, if you have not done so
already (by 10/12 please). The oral
presentations will be held in research conference
style at MIT's Endicott House estate in Dedham,
MA, on Saturday, October 18. WEB SITE
web.mit.edu/5.062/www/
2
Structural and Spin State Changes upon Binding of
Dioxygen to an Iron Porphyrin Center
Deoxy Hb (T state) Oxy Hb (R state). Hb
binds 4 O2 molecules. When 2 are bound, T
switches to R and makes the
next ones easier to
bind.
High-spin ferrous
Low-spin ferric
3
Model Chemistry for Oxy Hb and Oxy Mb
The problem FeIIP O2 FeIIIPO2-
PFeIIIO
OFeIIIP
..
FeIIP
..
..
FeIIP
2PFeIVO PFeIIIOFeIIIP
m-oxo, dimer
ferryl
The solutions Attach the porphyrin to a solid
support to avoid the bimolecular reaction or,
use low T, non-aqueous solvents, and py or 1-MeIm
complexes, but stability is lost at - 45 C or
above. The best solution was the construction of
a sterically hindered cavity for dioxygen binding
to avoid the intemolecular chemistry leading to
the thermodynamic sink of the system, the
(m-oxo)diiron(III) species.
4
Synthetic Models for OxyHb and OxyMb
(Collman)
(Baldwin)
5
The Cytochrome P-450 Reaction Cycle
When an axial site is available on the iron
porphyrin, dioxygen can bind and/or be activated
there. With proton-mediated reductive activation
of the O2 molecule, a peroxo intermediate forms
that converts to an FeIVO species, the ferryl
ion. The ferryl can oxidize hydrocarbons to
alcohols, epoxidize olefins, oxidize amines to
amine oxides and do related chemistry. P-450s
are liver enzymes necessary for metabolism and
used to convert pro-drugs and pro-carcinogens
to their active forms.
6
Protoctechuate 3,4-Dioxygenase
Notes dioxygenase vs. monooxygenase iron
oxidation state does not change iron acts as a
Lewis acid semiradical character of the
catecholate ligand activates it for
direct attack by the dioxygen molecule.
7
Hemerythrins - Diiron Dioxygen Carriers
Properties Mono- (myo Hr) and multi- (Hr)
subunit proteins. Found in marine invertebrates.
Easily isolated protein crystallizes after
one step!! Deoxy Hr, colorless, diiron(II) Oxy
Hr, red, diiron(III) peroxo nOO, 844 cm-1
in the terminally bound peroxide region.
nFeOFe, 486 cm-1, resonance enhanced symmetric
stretch. The asymmetric stretch occurs at
757 cm-1. Mixed-valent, semimet Hr,
Fe(II)Fe(III) inactive.
8
Structure of Azidomethemerythrin
Contains a (m-oxo)diiron(III) core. Met,
artificially oxidized. An inactive form of the
protein. The azido anion occupies the place of
the hydroperoxo anion in oxyHr. The structure was
encountered for the first time when the protein
crystallographers found it in azidometmyoHr. Myo,
single subunit. The electronic spectrum is
characteristic and a consequence of
antiferromagnetic spin exchange between the two
high-spin Fe(III) centers.
9
Note proton-coupled electron transfer Evidence
for proton transfer comes from resonance Raman
work
10
Early Structural Models for Methemerythrin
These and related complexes have no site for
binding of azide or dioxygen related species such
as hydroperoxide. The syntheses exemplify
spontaneous self-assembly. The challenges are to
make a site available, allow redox chemistry to
occur, and avoid polymerization to rust or
molecular ferric wheels and related complexes.
11
(No Transcript)
12
None does the chemistry of the protein!
13
Properties of Oxy Hr, Deoxy Hr, and Models
14
Structure and Chemistry of Class I Ribonucleotide
Reductase R2 Protein
Reaction of the reduced diiron(II) form of the R2
protein with dioxygen affords a high valent,
Fe(III)Fe(IV) intermediate designated as X.
Intermediate X is kinetically competent to
oxidize the tyrosyl residue to afford a tyrosyl
radical. This radical in turn transfers electrons
to the R1 subunit of the enzyme where a
Cys-SS-Cys cation radical forms. This radical in
turn initiates chemistry to convert ribo- to
deoxyribonucleotides.
15
Hemocyanins - Dicopper Dioxygen Carriers
Properties Multi-subunit proteins, ranging in
size up to 460 kDa. Found in spiny lobsters,
crayfish, and arachnids. Deoxy Hc, colorless,
dicopper(I) Oxy Hc, blue, dicopper(II)
peroxide nOO, 745-750 cm-1 in the peroxide
region, but low. Unusual structure, first
established by model chemistry
O Cu Cu
O
16
Schematic Views of Deoxy and Oxy Hc
Note, Type III copper
17
Structure of Deoxyhemocyanin
The two Cu atoms are held by six terminal
histidine residues, the Cu Cu distance being
3.7 Å. There is no obvious bridging ligand.
...
18
Monooxygenase Activity in Synthetic Cu2 Models
The dinuclear complex mediates insertion into the
CH bond. The chemistry mimics that of
tyrosinase.
19
Important Relationships
Reversible O2 binding
O2 Activation
- Iron porphyrin, Hb/Mb Iron
porphyrin, P-450 - Dicopper center, Hc Dicopper
center, tyrosinase - Diiron center, Hr
Diiron center, R2, MMO
WHAT CONTROLS THE FUNCTION??
20
Principles Illustrated by these Cases
Substrate binding and redox changes occur
- In all three cases, O2 binding is accompanied by
electron transfer from one or two metal ions to
dioxygen.
Coupled proton-electron transfer steps set the
potentials
- In oxyHr a proton transfers from the bridging
hydroxide to the peroxo ligand this step appears
to block further conversion to high-valent iron
oxidase center(s).
Metal center used to create or destroy radical
species
- Occurs in ribonucleotide reductase R2 protein.
Catechol dioxygenase - Fe(III) coordination
favors semiquinone form of a bound ligand without
redox reaction occurring.
Changes in metal coordination sphere facilitate
allostery
- Explains the cooperativity of O2 binding in Hb.
21
(No Transcript)
22
Methanotrophs are Used in Bioremediation of the
Environment
Prince William Sound, Alaska After the Exxon
Valdez oil spill, fertilizers were spread on the
beaches and natural methanotrophs restored their
pristine beauty.
Plants recruit oil-detoxifying microbes, as
discovered by scientists analyzing the recovery
of the environment in the Persian Gulf region
following the 1991 Gulf War. " In the root zone
was a rich reservoir of well-known oil eating
microbes... one family of which (Arthrobacter)
accounted for fully 95 percent..." Science News,
148, 84 (August 5, 1995)
23
The Mineral Springs in Bath, England, Source of
Methylococcus capsulatus (Bath)
The Restutive Contents of the WATERs Concoctive
Power Solution of gaffes, chaos of Salts and
mineral effluvia of subterranean expiration. It
cleanses the body from all blotches, scurvicial
itchings and BREAKING OUTS WHATSOEVER!
24
(No Transcript)
25
Structures of the sMMO Components