Principles%20of%20Bioinorganic%20Chemistry%20-%202003 - PowerPoint PPT Presentation
Title:
Principles%20of%20Bioinorganic%20Chemistry%20-%202003
Description:
The grade for this course will be determined by a term exam (35%), a written ... residues in the active site that position the substrate moiety for bond scission. ... – PowerPoint PPT presentation
Number of Views:48
Avg rating:3.0/5.0
Title: Principles%20of%20Bioinorganic%20Chemistry%20-%202003
1
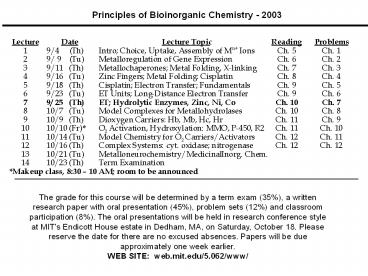
Principles of Bioinorganic Chemistry - 2003
The grade for this course will be determined by a
term exam (35), a written research paper with
oral presentation (45), problem sets (12) and
classroom participation (8). The oral
presentations will be held in research conference
style at MIT's Endicott House estate in Dedham,
MA, on Saturday, October 18. Please reserve the
date for there are no excused absences. Papers
will be due approximately one week earlier. WEB
SITE web.mit.edu/5.062/www/
2
Artificial Donor-Acceptor Pairs
Cytochrome c Fe---Ru, 12 Å
3
Method for Studying ET of Ru-Modified Proteins
Rate 30 s-1, T-independent
4
Distance and Driving Force Dependencies of ET
Rates
5
Driving Force Dependence
Data are from ruthenium-modified cytochrome c
derivatives (upper) and a series of covalently
linked donor/acceptor compounds
6
Distance dependence from the TDA term b from the
slope is 1.4 Å-1. Get a 10-fold decrease in rate
for every 1.7 Å increase in distance For
comparison, b for ET in vacuum is 2.8 Å-1 and b
for ET through covalent bonds is 0.7 Å-1
(thanks to Brian Crane for the plot)
7
The Mineral Springs in Bath, England, Source of
Methylococcus capsulatus (Bath)
The Restutive Contents of the WATERs Concoctive
Power Solution of gaffes, chaos of Salts and
mineral effluvia of subterranean expiration. It
cleanses the body from all blotches, scurvicial
itchings and BREAKING OUTS WHATSOEVER!
8
(No Transcript)
9
NMR Structure of the Fd Domain of MMOR Mueller,
Biochemistry, 41, 42-51 (2002)
10
(No Transcript)
11
Optical Spectra of MMOR and its Fd and FAD Domains
MMOR oxidized
Fd
FAD
Reduced forms
12
Redox States of the FAD Cofactor
13
Each trace is fit as a sum of exponentials giving
rise to the reported rate constants.
14
(No Transcript)
15
Summary - Points to Remember
- Three major metallic units transfer electrons in
bioinorganic chemistry iron-sulfur clusters
blue copper including the dinuclear CuA and
cytochromes (iron porphyrins). - Electrons can transfer over long distances in
10-15 Å hops . The rate depends on driving
force, distance, and orientation of the reacting
partners. Pathways are important (s gt p gt H-bonds
according to theoretical models). - Electron transfer within and between proteins is
optimized to take advantage of the molecular
switching stations. Included are organic units
such as flavins and inorganic units such as
iron-sulfur clusters, both used in the MMOR
protein.
16
Hydrolytic Enzymes, Zinc and other Metal Ions
PRINCIPLES
- M(OH)n centers supply OH- at pH 7 by lowering
water pKa - Mn serves as general Lewis acid, activating
substrates - Rate acceleration occurs by internal attack
within coord. sphere - Protein side chains greatly assist assembly of
transition state - Carboxylate shifts can occur, especially at
dimetallic centers - Electrostatic interactions predominate
- Non-redox active metal ions often but not
universally used
Illustrating the Principles
- Carboxypeptidase, carbonic anhydrase - delivering
hydroxide - Alcohol dehydrogenase an oxidoreductase
- Dimetallic metallohydrolases are two metals
better than one?
17
Carboxypeptidase A A Hydrolytic Zinc Enzyme
Reaction catalyzed
RCHC(O)NHR
RCHCO2- NH3R
NH2R
NH2R
Cleaves C-terminal peptide bonds prefers
aromatic residues.
Active site contains a single catalytic zinc,
essential for activity. The glutamate can undergo
a carboxylate shift. Thermolysin has a similar
active site it is an endopeptidase.
18
Carboxypeptidase A structure with the inhibitor
glycyl-L-tyrosine bound at the active site. Note
hydrogen bonds to key residues in the active site
that position the substrate moiety for bond
scission.
19
Catalytic Mechanism for Carboxypeptidase A
Summary of events 1. Substrate binds orients by
the terminal carboxylate. 2. Deprotonate bound
H2O. 3. Polarize scissile bond by Arg127. 4.
Bound OH- attacks peptide C(O). 5. Form
tetrahedral transition state. 6.Lose 2 peptide
fragments and recycle the enzyme. Principles
illustrated 1. Zinc serves as template. 2.Metal
supplies cleaving reagent, OH-, and organizes key
groups. 3. Chemistry achieved at neutral pH! Kcat
100 s-1 .
20
Carbonic Anhydrase, the First Known Zn Enzyme
Reaction catalyzed
CO2 H2O
H2CO3 106 s-1
21
Carbonic Anhydrase
PZn(OH2)2
PZn(OH) H
Keq 10-7M kf/kr
Note Rate 10-2 s-1 at pH 7 kf 106 s-1 in
active site. Paradox The reverse reaction is
diffusion controlled, with kr 1011 M-1 s-1
Thus kf 104 s-1. So how can the turnover be 106
s-1 ? Answer Facilitated diffusion of
protons by buffer components bound to the
enzyme.
22
Possible Carbonic Anhydrase Mechanism
23
Alcohol Dehydrogenase, an Oxidoreductase
Reaction catalyzed
RCH2 OH NAD
RCHO NADH H
Enzyme contains two 40 kDa polypeptides, each
with 2 Zn2centers in separate domains. One zinc
is structural, the other catalytic.
Catalytic zinc is 20 Å from the surface, near
the nicotinamide binding region. This center is
not required for NAD cofactor binding. Alcohol
substate DO require zinc and bind directly to the
metal center, displacing the coordinated water.
24
Schematic Diagram NAD binding to the active
site of LADH, with specific, well-positioned
amino acid side chains holding it in place.
Ethanol is shown bound to the zinc, displacing
water. The system is set to undergo catalysis.
25
Note hydride transfers from a-C of alcohol to
nicotinamide ring.
26
(No Transcript)
27
Dimetallics can move the value into the
physiological range near pH 7
28
Advantages of Carboxylate-Bridged Dimetallic
Centers in Chemistry and Biology
29
(No Transcript)
30
(No Transcript)
31
(No Transcript)
32
(No Transcript)
33
(No Transcript)
34
Alkaline Phosphatase a Dizinc(II) Center
Activates the Substrate
1. The substrate binds to the dizinc center a
nearby Arg also helps activate it. 2. A serine
hydroxyl group attack the phosphoryl group,
cleaving the ester. The phosphate is transferred
to the enzyme, forming a phosphoryl-serine
residue. 3. Hydrolysis of this phosphate ester by
a zinc-bound hydroxide com-pletes the catalytic
cycle. This mechanism is supported by studies
with chiral phosphate esters (ROP18O17O16O)2-
there is no net change in chirality at phoshorus.
1.
3.
2.
35
Principles illustrated the dimetallic affords
hydroxide the substrate is positioned by
residues in the active site the dimetallic
stabilizes the urea leaving group redox inactive
metal electrostatics
36
(No Transcript)
37
(No Transcript)
38
(No Transcript)
39
(No Transcript)
40
Metallo-b-lactamases, an Emerging Clinical
Problem
PZn(OH2)2
PZn(OH) H
Keq 10-7M kf/kr
41
b-Lactamase from Bacteroides fragilis
N.O. Concha, B.A. Rasmussen, K. Bush, O. Herzberg
(1996), Structure 4, 823-836
42
(No Transcript)
43
(No Transcript)
44
Summary - Points to Remember
- Both mono- and dimetallic centers lower the pKa
value of bound water, allowing hydroxide to be
delivered at pH 7. - Coordination of the leaving group portion of the
substrate to a metal ion activates the substrate
for nucleophilic attack. - Residues not coordinated but in the second
coordination sphere can participate directly
(serine in phophatases) or indirectly (arginine
in alcohol dehydrogenase) in substrate attack,
orientation, and/or activation. - Carboxylate shifts facilitate substrate binding,
activation. - Redox inactive metal ions (Zn2, Ni2, Mn 2,
Co2) preferred.