Chapter 5a Cell Respiration - PowerPoint PPT Presentation
1 / 20
Title:
Chapter 5a Cell Respiration
Description:
Chapter 5a - Cell Respiration. Cell respiration is a decomposition pathway that provides the ... Some similarities of Photosynthesis and cellular respiration ... – PowerPoint PPT presentation
Number of Views:43
Avg rating:3.0/5.0
Title: Chapter 5a Cell Respiration
1
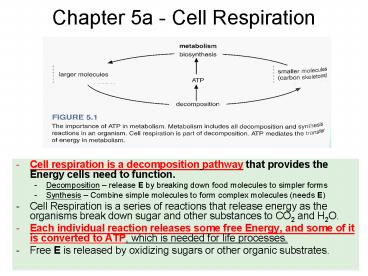
Chapter 5a - Cell Respiration
- Cell respiration is a decomposition pathway that
provides the Energy cells need to function. - Decomposition release E by breaking down food
molecules to simpler forms - Synthesis Combine simple molecules to form
complex molecules (needs E) - Cell Respiration is a series of reactions that
release energy as the organisms break down sugar
and other substances to CO2 and H2O. - Each individual reaction releases some free
Energy, and some of it is converted to ATP, which
is needed for life processes. - Free E is released by oxidizing sugars or other
organic substrates.
2
Aerobic and Anaerobic respiration
- Aerobic Respiration (with Oxygen present)
- - O2 is the oxidizing agent
- - O2 receives e- from the decomposed substrates
(complex molecules) - Anaerobic respiration (no Oxygen present)
- - A nitrogen or sulfur compound substitutes for
O2 - - The substrate may only be partially
decomposed, releasing less E - __________________________________________________
______
3
Cell respiration versus Breathing
- Not the same thing, but they do have a
relationship - Breathing ( exchange of gases CO2 and O2
between the external environment and the
organism). Various organisms have various
structures for this (body surface, gills, lungs,
stomata, etc.) - Cell respiration (Breaking down complex molecules
as source of E, removal of waste CO2 excess
water from each individual cell, and renewal of
O2). - ____________________________________________
- - All organisms respire (use the chemical
reactions of cell respiration). - - Not all organisms use breathing in the same
sense as mammals use their lungs for gas
exchange. It depends on the specific structures
available for different organisms. - - Some organisms dont require oxygen, but they
do cell respiration to obtain Energy from complex
molecules, and to remove cellular waste.
4
Aerobic Respiration- Raw materials
- 1) Carbohydrates (sugars) The main sources of
Energy for cell respiration. - Glucose C6H12O6, and
- Glucose-Phosphate C6H11O6 H3PO3
- - Animals Produce them by digesting
carbohydrates (from food), or breaking down
Glycogen (animal starch, a complex
polysacharide) stored in liver or muscles. - - Plants Produce them by breaking down sucrose
and starch (complex storage polysacharide) - 2) Fats (lipids) Used for special needs (see
later) - 3) Proteins Used for special needs (see later)
- __________________________________________________
____ - enzymes
- C6H12O6 6O2 -------------? 6CO2
6H2O Energy - Glucose oxygen
carbon dioxide water
5
Products of cell respiration
- By breaking down raw materials during cell
respiration, 2 main products are made - 1) Carbon skeletons needed in biosynthesis of
other molecules, and - 2) ATP needed to fuel cellular processes.
6
Crash course on oxidation / reduction
- Oxidation
- - Removal of an e- from a molecule
- - Losing a hydrogen ion (H proton). (This is
the most frequent case in biological systems) - Reduction
- - Gaining an e-
- - Gaining a hydrogen ion (H).
- ___________________________________
- When a molecule is oxidized, the resulting e- and
H will be accepted by another molecule. Thus,
whenever a molecule is oxidized, another must be
reduced.
7
Aerobic respiration The lineup of players
- 1- Glucose (6-C, C6H12O6) broken down
- 2- 3-Carbon molecules formed
- 3- CO2 released
- 4- O2 must be present for some stages
- 5- 2-Carbon molecules formed
- 6- NAD (nicotinamide adenine dinucleotide)
reduced to NADH, then regenerated - 7- FAD (flavin adenine dinucleotide) reduced to
FADH2 - 8- H2O formed
- ___________________________________
- ATP is formed at each decomposition reaction
- NADH and FADH2 (reduced compounds) they carry
hydrogen ions (protons H) and e- to the
electron transport system.
8
Aerobic respiration 3 stages
- Glycolysis (Breakdown of
- sugars). Partial ? oxidation of
glucose (6-C) by enzymes, splitting it into 2 3-C
molecules. ATP is produced. - 2) Krebs Cycle. 1 CO2 ? molecule is
released in the complete oxidation of each of the
2 3-C molecules. An enzyme is involved, and more
ATP is formed.
9
Aerobic respiration 3 stages
- 3) Electron transport system. Synthetizes most of
the ATP generated during respiration. Receives
the Energy of protons (H) and e- from each
oxydation. H and e- are transferred to O2,
forming H2O. - 3)?
10
Some similarities of Photosynthesis and cellular
respiration
- Hydrogen-carrying molecules in cells
- - NADPH (from photosynthesis)
- - NADH, FADH2 (from respiration)
- All move H in cells
- All are oxidized and reduced in cycles, changing
from the oxidized form (with superscript ), to
the reduced form (with H at the end). - At the end of the electron transport systems, the
H carried by them will reduce oxygen to form
water molecules.
11
Where does cell respiration take place?
- Complex cells
- Bacteria
Multicellulars - __________________________________________________
_________ - e-transport Cell membrane Cristae of
- System (most Mitochondria
- ATP synthesis)
- __________________________________________________
- Krebs Cycle Cytoplasm Matrix of Mitochondria
- __________________________________________________
- Glycolysis, Cytoplasm Cytoplasm
- Fermentation
- __________________________________________________
- Mitochondria The powerhouses of the cell
- - Membrane- double layer as in all cells
- Inner layer many folds (cristae)
- - Matrix Fluid filled
- In Complex cells, the number of mitochondria is
related to the energy expenditure of each type of
cells, ranging from a few to several thousand.
12
Glycolysis (from glucose to pyruvate)
- 1 Glucose is converted to Glucose-6-phosphate (1
ATP used, enzyme involved) - Glucose-6-phosphate changed to diphosphate (gains
1 more P)(1 ATP used, another enzyme) - G-6-diphosphate splits into two 3-C
sugar-phosphates - Partial oxidation of two 3-C molecules to form
two 3-C pyruvic acid molecules NAD reduced to
NADH (2 ATP formed per each 3-C molecule) - In plants, Glucose or Glucose-P can enter in step
a), or 3-C sugar-P can enter in step c). - In animals, Glucose enters as in diagram.
- _____________________________
- Results (per molecule of Glucose)
- 4 ATP (but 2 were spent),
- 2 NADH,
- 2 pyruvate,
- Carbon skeletons
13
Glycolysis- Net results and fate of Pyruvate
- Net results of Glycolysis (from each Glucose)
- - 2 ATP
- - 2 NADH
- - 2 Pyruvate
- - Carbon skeletons
- ___________________________________
- Glycolysis begins both aerobic and anaerobic cell
respiration - Fate of Pyruvate
- With sufficient O2, the next step is the Krebs
Cycle (Aerobic, the most efficient method) - Without sufficient O2, the next step is
fermentation (Anaerobic, less efficient). - In animals, if insufficient O2, the process is
lactic acid fermentation - - NADH changes to NAD and goes back to
glycolysis, but ending in less ATP production - - Pyruvate changes to Lactate.
14
The Krebs Cycle (from pyruvate to CO2)
- (prior to Krebs Cycle) 2 Pyruvate enter
mitochondria. Enzymes take 1 CO2 from each,
changing them to acetate (2-C organic acid). 1
NAD reduced to NADH. CoA (Coenzyme A, an acetate
carrier molecule) binds to acetate, becoming
Acetyl-CoA and carries it to Krebs cycle. - Acetyl-CoA brings acetate. CoA and acetate are
separated. CoA is released and reused. Enzyme
combines acetate with oxaloacetate (4-C acid) to
form citrate (6-C acid). - c-d) Citrate is oxidized in several steps,
releasing CO2, and H atoms which reduce 2 NAD to
2 NADH, and a 4-C organic acid. - e-f) 4-C organic acid is oxidized, resulting in
a new oxaloacetate that enters a new round of the
Krebs Cycle. 1 NAD is reduced to NADH and 1 FAD
is reduced to FADH2, and 1 ATP formed.
15
The e- transport systemSynthesis of ATP
- ATP is synthesized in mitochondria and released
to be used by the cell. - NADH and FADH2 carry H atoms into e- transport
system. - Enzymes and Cytochromes (proteins) in cristae
separate H into H and e-. Electrons are
transported step by step thru the system, each
step releasing free energy that drives the
synthesis of ATP by ATP synthetase enzyme complex
. - Each NADH can produce 3 ATP each FADH2 can
produce 2 ATP. - At the end, H2O is formed by a terminal
cytochrome, which reduces O2 with e- and H from
NADH and FADH2 (only step requiring oxygen).
16
Summary of aerobic respiration
- As glucose is oxidized in glycolysis and Krebs
cycle, NAD and FAD are reduced to NADH and FADH2,
and pass e- to the electron transport system. - H20 is formed by reduction of O2 with e- and H
from NADH and FADH2. - NAD and FAD are recycled and re-enter respiration
in the oxidation of more glucose. - For each glucose molecule entering aerobic
respiration, up to 38 ATP are produced (8 from
glycolysis, 6 from pyruvate to Acetyl CoA, 24
from Krebs cycle).
17
Review of molecules involved
- Molecule What is it ? How it
participates When - Kinese enzyme cascade series of enzymes make
glucose from glycogen - or starch before g.lys
- Glucose 6-C sugar broken down
(oxidized) glycolysis - Glucose-6-phosphate 6-C sugar
phosphate formed glycolysis - Pyruvic acid 3-C acid formed (with
O2) glycolysis - Pyruvate 3-C sugar formed (with
O2) end of g.lysis - NAD, NADH H carrier carries H atoms
(e-, H) g.lysis, Krebs - FAD, FADH2 H carrier carries H atoms
(e-, H) Krebs - ATP storage of free E formed,
spent g.lysis, Krebs - Lactic acid 3-C acid formed (if no
O2) glycolysis - Lactate 3-C sugar formed (if no
O2) end of g.lysis - Ethanol (ethyl alcohol) alcohol formed (if
no O2) bacteria - Acetic acid (vinegar) acid formed (if no
O2) bacteria - Acetate 2-C organic acid formed pre-Kre
bs - CoA (Coenzyme A) carrier enzyme binds to
acetate pre-Krebs - Acetyl CoA complex of CoAacetate delivers
acetate pre-Krebs - Oxaloacetate 4-C acid
- (complex of AcetylCoAacetate)
formed enters Krebs
18
Bacteria do it different !
- They only have 1 cell and dont have
mitochondria. - Glycolysis and Krebs Cycle take place in the
cells cytoplasm. The electron transport system
is located in the cell membrane. - Some bacteria (not all !!!) are anaerobic the
oxidizing agent is not oxygen but another
compound. They dont reduce O2 to form water, but
produce other reduced sulfur or nitrogen
compounds (H2S, NH3). - Types of bacteria depending on O2 utilization
- - Facultative Aerobes Depending on O2
availability, they can switch back and forth
between fermentation (anaerobic) and aerobic
respiration. - - Obligate Anaerobes Cannot live in the
presence of O2. They generate ATP from
fermentation or anaerobic respiration. - __________________________________________________
______ - Most higher organisms are Obligate Aerobes they
require O2 for most processes and cannot survive
long without it.
19
Linking Cell Respiration Photosynthesis
- Oxygen is needed to oxidize glucose (not for the
initial lysis). - - Without O2, pyruvate from glucose must be
fermented (anaerobically), a less efficient
process that produces less ATP. - - With O2, pyruvate enters Krebs Cycle,
producing more ATP. Thus, animals gain
proportionally more E from food than anaerobes. - Hydrogen carriers (NADP from Photosynthesis) and
NAD, FAD (from respiration) help cells catch free
energy into ATP molecules. - O2 for the e- transport system of aerobic
respiration comes from gas exchange organs
(lungs, stomata, gills), and distributed with
circulation systems. - Raw materials
- - Cell respiration O2 and carbohydrates
(from photosynthesis) - - Photosynthesis CO2 and water (from
respiration) - Both Respiration and photosynthesis produce
carbon skeletons for biosynthesis.
20
Cell Respiration Fill all the boxes (24)