Other Sources of Enthalpy Data - PowerPoint PPT Presentation
1 / 40
Title:
Other Sources of Enthalpy Data
Description:
... shown in the figure below was used for the separation of 0.25 mole pentane from heptane. ... if the feed condition changes from superheated to sat. vapor ... – PowerPoint PPT presentation
Number of Views:31
Avg rating:3.0/5.0
Title: Other Sources of Enthalpy Data
1
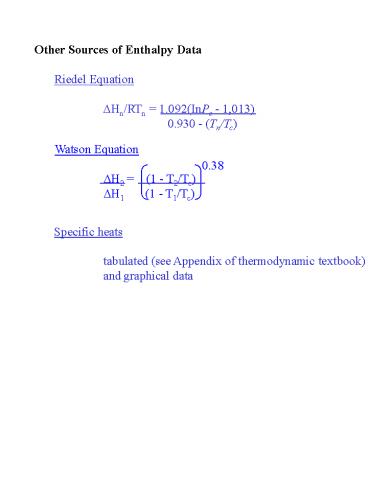
Other Sources of Enthalpy Data
Specific heats tabulated (see Appendix of
thermodynamic textbook) and graphical data
2
Example 14b Calculate QC using Riedel and Watson
Equations. What would be the value of QC if the
distillate subcools liquid to 30C?
3
Specific Heats of Liquids
4
Specific Heats of Gases
5
Internal Column Balances
Enriching section of the column
6
Example 14c What is the composition and enthalpy
of vapor entering and leaving the first tray of
the distillation column shown in example 14a.
7
External Column Balances
Stripping section of the column
8
Lewis Method
Important assumptions in distillation column
calculation (1) column is adiabatic (2) specific
heat ltlt latent heat (3) latent heat (l) is
constant independent of concentration this
means one mole of condensed vapor will evaporate
1 mole of liquid (4) saturated liquid and vapor
lines in H-x-y diagram are parallel
9
Lewis Method
Vi, yi
(i)
Equilibrium relation y Kx
Li, xi
Vi1, yi1
Operating equation rectifying section yj1
(L/V)xj (1-L/V)xD stripping section yk
(L/V)xj - (L/V-1)xB
10
Example 15 What is the composition and enthalpy
of vapor entering and leaving tray 1-5 of the
distillation column shown below.
11
VLE Data
12
External Column Balances
13
McCabe-Thiele Method
14
Example 16 What is the composition and enthalpy
of vapor entering and leaving tray 4-9 plus the
reboiler for the distillation column shown
below.
15
VLE Data
16
McCabe-Thiele Method
17
Example 17 A mixture of pentane and toluene was
distilled in a distillation column. An analysis
of the enriching section is needed to determine
whether the column is performing to specification.
partial condenser
D, yD 0.9 hD, R 2
V1
L0
(1)
Please determine the composition of liquid and
vapor streams leaving each stages for (a) a 2
and 3.5 with R 2, (b) a 3.5 with R 1 and 4
(2)
(3)
V4
L3
18
McCabe-Thiele Method
a 2
a 3.5
19
McCabe-Thiele Method
a 3.5
a 3.5
20
Total and Minimum Reflux
Total Reflux D 0, L0 V1, R
L0/D ? L/V L0/V1 1
Minimum Reflux D maximum, L0 minimum
allowable,
a 3.5
21
Example 18 Analysis of the stripping section of
the distillation column for pentane-toluene
separation must be conducted to determine the
liquid and vapor compositions leaving each
distillation trays.
(a) using a total reboiler, (b) using a partial
reboiler.
22
McCabe-Thiele Method
a 3.5
a 3.5
23
Internal Column Balances
Feed tray
Feed Equation
y -(L - L)/(V - V)x Fzf/(V-V) y
-(Lf/Vf)x (F/Vf)zf y q/(q-1)x
zf/(1-q) q (L-L)/F (H-hf)/(H-h)
24
Example 19 Find the value of q and draw the feed
line for a feed containing 0.4 pentane and 0.6
toluene
(a) the feed is a saturated liquid, (b) the feed
contains 0.5 fraction of vapor, (c) the feed was
superheated so that each mole of feed vaporizes
10 moles of liquid, (d) the feed was subcooled so
that each mole of feed condenses 2 moles of vapor.
25
McCabe-Thiele Method
a 3.5
a 3.5
26
McCabe-Thiele Method
a 3.5
a 3.5
27
Example 20 The distillation column shown in the
figure below was used for the separation of 0.4
mole fraction pentane in toluene. The desired
distillate and bottom products are 0.1 and 0.9,
respectively. The feed enters the column as a
superheated vapor that vaporizes 2 moles of
liquid per mole of feed.
(a) What is q-value of the feed? Plot the feed
line. (b) What is the minimum reflux ratio for
the separation? (c) If the column reflux was
operated at 3 Rmin, where is the optimum
feed-plate location?
(d) What is the boil-up ratio needed for the
separation? (e) How many equilibrium stages is
needed to accomplish the desired separation? (f)
How much distillate and bottom are produced if
the feed rate is 10 kmole/min? (g) What is the
minimum number of trays needed for achieve the
desired separation?
28
McCabe-Thiele Method
a 3.5
a 3.5
29
Example 21 The distillation column shown in the
figure below was used for the separation of 0.25
mole pentane from heptane. The desired distillate
and bottom products are 0.05 and 0.95,
respectively. The vapor flowrate in the enriching
and stripping sections of the column are 2 D and
3B, respectively.
(a) What are the flowrates of distillate and
bottom? (b) What is the (L/V)enriching and plot
the top operating line? (c) Express the the
operation reflux ratio, R as n Rmin
(d) What is the boil-up ratio? Plot the bottom
operating line. (e) Is the feed subcooled,
saturated liquid, mixture, saturated vapor or
superheated vapor? (f) How many equilibrium
stages is needed to accomplish the desired
separation? (g) Where is the optimum location of
the feed plate?
30
McCabe-Thiele Method
a 2
a 2
31
Example 22 A stripping column shown in the
figure below was used to remove oil from
contaminated water. The water leaving the bottom
must be at least 99.7 pure. The VLE data is
plotted in the figure below.
32
McCabe-Thiele Method
a 3.5
a 3.5
33
McCabe-Thiele Method
a 3.5
a 3.5
34
Example 23 Crude oil could be extracted from
sand found in Canadian province of Saskatchewan.
Steam is used in the extraction process and the
oil-water mixture is send through a series of
distillation column. The final column known as
the dehydrating column is employed for removing
the final traces of water from the crude to meet
the industrial maximum tolerance level of 0.01
mole fraction water. Instead of a condenser
saturated liquid water was used directly as
coolant. This arrangement has the added benefit
of diluting the oil that remains in the water
recovered at the distillate. The water from the
distillate is then sent to settling tank to
remove the final traces of oil before discharge.
D, yD 0.8, hD
(a) Derive the top operating equation for
dehydration column. (b) Derive the bottom
operating equation (c) Derive the feed
equation (d) Plot the respective top and bottom
operating line as well as the feedline
Q0
(n)
Reboiler
QR
B, xB 0.01, hB
Boilup ratio Vn1/D 3
(e) Determine the number of stages needed for the
separation and the optimum feed plate location if
the total tray efficiency is 0.25.
35
McCabe-Thiele Method
a 3.5
a 3.5
36
Example 24 The distillation column shown in the
figure below was used for the separation of 0.5
mole fraction methanol-water solution. The
desired distillate and bottom products are 0.10
and 0.95, respectively. The feed enters the
column as a superheated vapor that vaporizes 2
moles of liquid per mole of feed.
(a) What is q-value of the feed? Plot the feed
line. (b) What will happen if the feed condition
changes from superheated to sat. vapor to sat.
liquid and subcooled liquid.
(c) What is the minimum reflux ratio? (d) What is
the minimum number of plates?
37
McCabe-Thiele Method
a 3.5
a 3.5
38
Column Efficiency
Overall Column Efficiency
Eo Nequil/Nactual
Murphree Efficiency
EMV actual change in vapor
change in vapor for equilibrium stage
yj - yj1
yj - yj1
39
Example 25 The distillation column shown in the
figure below was used for the separation of 0.5
mole fraction methanol-water solution. The
desired distillate and bottom products are 0.20
and 0.9, respectively. The feed enters the column
as a subcooled liquid that condenses 2 moles of
vapor per mole of feed.
(a) What is q-value of the feed? Plot the feed
line. (b) What is the number of equilibrium
stages? (c) What is the actual number of stages
if the EMV 0.5? (d) Solve the problem using
Fenske, Gilliland and Underwood methods.
40
McCabe-Thiele Method
a 3.5
a 3.5