PowerPointpresentasjon - PowerPoint PPT Presentation
1 / 1
Title:
PowerPointpresentasjon
Description:
Department for Inspection and Narcotic Drugs Control. Elisabeth ... S.f. Administrative functions. S.M. Wold. S.f. Pharmaceutical. Evaluation. A-K. Rolstad ... – PowerPoint PPT presentation
Number of Views:59
Avg rating:3.0/5.0
Title: PowerPointpresentasjon
1
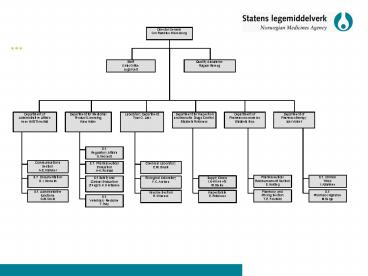
Director General Gro Ramsten Wesenberg
Staff Outer Office Legal Unit
Quality AssuranceRagnar Røreng
Department of Administrative Affairs Anne Britt
Thuestad
Department for Medicinal Product Licensing Hans
Halse
Laboratory Department Tove G. Jahr
Department of Pharmacotherapy Ivar Vollset
Department for Inspection and Narcotic Drugs
Control Elisabeth Rokkones
Department of Pharmacoeconomics Elisabeth Bryn
S.f. Regulatory Affairs S. Woxholt
Communications Section A-E. Hammer
S.f. Pharmaceutical Evaluation A-K. Rolstad
Chemical Laboratory E.M. Brevik
S.f. Clinical Trials I. Aaløkken
S.f. DocumentationB. I. Norheim
Pharmaceutical Reimbursement Section E. Hviding
S.f. Safety and Clinical Evaluation (Fung) S.K.
Gustavsen
Supply Chain, Licences etc M. Bjerke
Biological Laboratory F.C. Arntzen
S.f. Administrative functions S.M. Wold
Pharmacy and Pricing Section T.E. Frostelid
S.f. Pharmacovigilance M.Berge
Vaccine Section R. Winsnes
Inspectorate E. Rokkones
S.f. Veterinary Medicine T. Høy