Substituents PowerPoint PPT Presentations
All Time
Recommended
Carbonic Acid. HOCOH. O. CO2. H2O. HOCO. O. H overall K for ... CO2 is major species present in a solution of 'carbonic acid' in acidic media. Carbonic Acid ...
| PowerPoint PPT presentation | free to download
Title: Organic Chemistry Fifth Edition Subject: Sections 12.12-12.14 Author: Frank Carey Last modified by: smpibm Created Date: 8/15/2000 4:05:16 PM
| PowerPoint PPT presentation | free to view
19.6. Substituents and Acid Strength. standard of comparison is acetic acid (X = H) ... sp2-hybridized carbon is more electron-withdrawing than sp3, and sp is more ...
| PowerPoint PPT presentation | free to view
... are meta directing and strongly deactivating. Many EWGs Have a Carbonyl Group ... the net effect is that halogens are deactivating but ortho-para directing ...
| PowerPoint PPT presentation | free to view
Impact of substituents on the metal-based redox potential for a series of complexes based on trans-[Cl(pyridine)4Ru-L]+ where L is a para-substituted derivative of ...
| PowerPoint PPT presentation | free to download
... there must be strong electronic conjugation between the substituent groups and ... in COR-2, the conjugation between fluoromethyl groups and tetrapyrrole ...
| PowerPoint PPT presentation | free to view
Mesitylene (or 1,3,5-trimethylbenzene) is a derivative of benzene with three methyl substituents symmetrically placed on the ring.
| PowerPoint PPT presentation | free to download
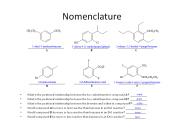
Nomenclature What is the positional relationship between the two substituents in compound A? _____ What is the positional relationship between the two substituents ...
| PowerPoint PPT presentation | free to download
Saturated hydrocarbons Chapter 12 Branched-chain alkyl groups Given a choice between unbranched substituents and branched substituents, use unbranched ones for naming ...
| PowerPoint PPT presentation | free to download
R substituents strengthen acids and weaken bases. X. Y ... acyloxy. dialkylamino. alkyl. chloro. iodo. Y. Substituents with Electron-Withdrawing ...
| PowerPoint PPT presentation | free to view
Glutathione injections in Dubai are the natural solution for skin whitening. They are chemical-free, made of natural substituents, improve the skin tone by boosting the immune system.
| PowerPoint PPT presentation | free to download
Substituent Effects on Electrophilic Aromatic Substitution meta-Directing Substituents ortho-para-Directing Groups ... that the nitro substituent is a meta director.
| PowerPoint PPT presentation | free to view
Carbon atoms bearing four different substituents are called asymmetric centers ... Chiral compounds with one stereocenter exist as two ... The polarimeter ...
| PowerPoint PPT presentation | free to view
Electronegative substituents promote formation of the carboxylate ion. 11 ... Multiple electronegative substituents have synergistic effects on acidity. 12 ...
| PowerPoint PPT presentation | free to view
Always determine the longest chain whether bent or straight. ... Name substituents and list in ABC order. Number substituents ...
| PowerPoint PPT presentation | free to view
Weakly deactivating substituents: (halogens) Moderate to strongly deactivating substituents: (carbonyl ... c. X deactivates the ring and directs para/ ortho. ...
| PowerPoint PPT presentation | free to download
... Directing effects of substituents already on the ring. Products of Nitration ... Substituents that make the ring react faster (than benzene) with electrophiles ...
| PowerPoint PPT presentation | free to view
... acrylamides, acrylic acid, acrolein, vinyl ketones vinyl epoxides perfluorinated alkane olefins styrenes (large ortho substituents) ...
| PowerPoint PPT presentation | free to view
Phenols 24.1 Nomenclature Nomenclature named on basis of phenol as parent substituents listed in alphabetical order lowest numerical sequence: first point of ...
| PowerPoint PPT presentation | free to view
Olefin Metathesis allows the exchange of substituents between different olefins ... The Ring-Closing Metathesis (RCM) allows synthesis of 5- up to 30-membered ...
| PowerPoint PPT presentation | free to view
Free Research Report PDF: https://bit.ly/3bqYKhX If hydrocarbon substituents are bound to the silicon atoms, the polymers are designated as Organopolysilazanes. Molecularly, polysilazanes [R1R2Si-NH]n are isoelectronic with and close relatives to Polysiloxanes [R1R2Si-O]n (silicones). Download Free Research Report PDF: https://bit.ly/3boAMUJ
| PowerPoint PPT presentation | free to download
High hydrophobicity. Activity of drugs is often related to P ... substituent hydrophobicity constant. A measure of a substituent's hydrophobicity relative to ...
| PowerPoint PPT presentation | free to view
(steric) Energy Match. Orbitals of the same electronegativity form better. covalent bonds. ... Steric. Big substituents can affect geometry. CH3 rvdw = 200 pm ...
| PowerPoint PPT presentation | free to view
1) Benzene is last in the name. 2) List substituents alphabetically. Number ring in direction that gives lowest. locant at first point of difference ...
| PowerPoint PPT presentation | free to view
As a substituent - phenyl. Nomenclature of Benzene. Two Substituents. Nomenclature of Benzene ... If a common aromatic that sub gets #1 otherwise number to give ...
| PowerPoint PPT presentation | free to view
Substructure allows substituents at vacant positions ... Do substructure search (autofix OK) Get References - reactant. Display. Build reaction query ...
| PowerPoint PPT presentation | free to view
When 0 r 1, the reaction is less sensitive to substituents ... P. Jr. Wentworth, A. Datta, S. Smith, A. Marshall, L. J. Partridge, and G. M. Blackburn, J. Am. ...
| PowerPoint PPT presentation | free to download
To obtain pure modified-Hantzsch products with various substituents on the ... nifedipine, is an effective drug but has some undesirable clinical features. ...
| PowerPoint PPT presentation | free to view
Find longest carbon chain. Base name is same as n-alkane of that length. ... Different substituents list in alphabetical order ...
| PowerPoint PPT presentation | free to view
The cis isomer must have one substituent in an axial position and one in an equatorial position ... The trans isomer has both substituents in either the ...
| PowerPoint PPT presentation | free to view
Find the longest continuous carbon chain, and this defines the name of the alkane. Number the carbons, starting closest ... Arrange substituents alphabetically. ...
| PowerPoint PPT presentation | free to view
Constitutional Isomers ... The cis isomer, has two substituents on the same side of the ring. ... Cis-Trans Isomers. The compound 1,2-dichlorocyclopropane has ...
| PowerPoint PPT presentation | free to view
Stereoisomers arise when there is a chiral carbon. Chiral carbon- four different substituents bonded ... Epimers- differ in arrangement about one other chiral carbons. ...
| PowerPoint PPT presentation | free to view
Download FREE Sample Report- http://bit.ly/2vdpDn7 Silanes are monomeric silicon compounds with four substituents, or groups, attached to the silicon atom. These groups can be the same or different and nonreactive or reactive, with the reactivity being inorganic or organic. Inorganic reactive silanes have alkoxysilane groups and undergo hydrolytic polycondensation reactions. Organic reactive silanes contain amine, vinyl, epoxy, isocyanate or other functional groups that enable reactions with organic functionalities. for more visit here- http://bit.ly/2Dlgopf
| PowerPoint PPT presentation | free to download
Predict the major product of the following reaction: ... A nucleophile replaces a leaving group on the aromatic ring. Electron-withdrawing substituents activate ...
| PowerPoint PPT presentation | free to view
directing effects of substituents reinforce. each other; ... Presence of electronegative atom (N) in ring. causes electrons to be held more strongly than ...
| PowerPoint PPT presentation | free to view
General Molecular Formula for Alkenes. Each bond introduced, reduces the H ... that are used as names for substituents are the vinyl group and the allyl group ...
| PowerPoint PPT presentation | free to view
Draw any Fischer Projection of any chiral molecule. Rank substituents as in R/S nomenclature ... gives major product when several. stereoisomers are possible ...
| PowerPoint PPT presentation | free to view
More stable since the double bond. bears more substituents. Less ... Diene dienophile adduct. 7. Diene dienophile adduct. Either diene or dienophile must have ...
| PowerPoint PPT presentation | free to view
Examples of molecules with 1 chirality center. Chiral as a result of isotopic substitution ... the substituents at the chirality. center according to same rules ...
| PowerPoint PPT presentation | free to view
HOMO-LUMO energy gap is affected by substituents on double bond ... conjugated triene plus two methyl groups. Lycopene. max 505 nm. orange-red pigment in tomatoes ...
| PowerPoint PPT presentation | free to view
Wherever H has been replaced what is replacing it is considered a substituent ... Same substituents indicate number of with prefix: di, tri, tetra, penta, etc. ...
| PowerPoint PPT presentation | free to download
Active Cl species include Cl2, HOCl, and ClNO2 ... The Cl and/or F substituents lend HCFCs some of the desirable properties of CFCs ...
| PowerPoint PPT presentation | free to view
The most abundant biological molecules. Compounds of the generic formula (C.H2O)n (n3) ... Predominates -Position of bulkier substituents. Axial. Sugar Derivatives ...
| PowerPoint PPT presentation | free to view
A series of compounds in which the members are built up in a regular, repetitive ... Alkyl or halogen substituents attached to the rings are named similarly to alkanes ...
| PowerPoint PPT presentation | free to view
Substituents that withdraw electrons from the benzene ring will destabilize the ... a pale yellow solid, melts at 83 C, and explodes at 240 C. Supplying TNT with ...
| PowerPoint PPT presentation | free to view
Number from end closest to double (triple) bond. Name and number of substituents BEFORE ... 'If it crawls, it's biology. If it doesn't work, it's physics. ...
| PowerPoint PPT presentation | free to view
Indicate halogen substituents by the prefixes fluoro-, chloro-, bromo-, and iodo ... Locate each halogen on the parent chain by giving it a number preceding the name ...
| PowerPoint PPT presentation | free to view
The two substituents will modify the crystalline lattice and finally will affect ... Reflection spectra were used to show the effect of the donor on the oxidation ...
| PowerPoint PPT presentation | free to view
Organic Chemistry 6th Edition Paula Yurkanis Bruice Chapter 16 Reactions of Substituted Benzenes *
| PowerPoint PPT presentation | free to download
... energy: 44.35kcal/mol Tingbin Wen, Guochen Jia. Angew. Chem. Int. Ed. 2001, 40,1951 Guochen Jia, Zhenyang Lin. Organometallics, 2003,22,3898.
| PowerPoint PPT presentation | free to view
Title: 16_Lecture.ppt Author: Edward Skibo Last modified by: Melissa Renfroe Created Date: 3/31/2003 7:00:51 PM Document presentation format: On-screen Show
| PowerPoint PPT presentation | free to view
Title: PowerPoint Presentation Author: Michael W. Justik Last modified by: WRDSB Created Date: 1/18/2005 6:12:23 PM Document presentation format: On-screen Show (4:3)
| PowerPoint PPT presentation | free to download
Carboxylic Acids: Properties and Synthesis Assignments In-text problems: 18-1 and 18-2 18-4 ... Electron-withdrawing effects involving resonance (-R) ...
| PowerPoint PPT presentation | free to view
Simple Organic Chemistry Basic Structure and Nomenclature Graphic: www.lab-initio.com First Ten Alkanes Formula Name Formula Name CH4 Methane C6H14 Hexane C2H6 Ethane ...
| PowerPoint PPT presentation | free to view
... with either isotactic or the syndiotactic configuration can be prepared using an aluminum titanium initiator (Ziegler Natta catalyst): ...
| PowerPoint PPT presentation | free to view





![Impact of substituents on the metal-based redox potential for a series of complexes based on trans-[Cl(pyridine)4Ru-L] where L is a para-substituted derivative of cyanobenzene PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/6860403.th0.jpg)