Aromatic Substitution - PowerPoint PPT Presentation
1 / 27
Title:
Aromatic Substitution
Description:
Substituent Effects on Electrophilic Aromatic Substitution meta-Directing Substituents ortho-para-Directing Groups ... that the nitro substituent is a meta director. – PowerPoint PPT presentation
Number of Views:139
Avg rating:3.0/5.0
Title: Aromatic Substitution
1
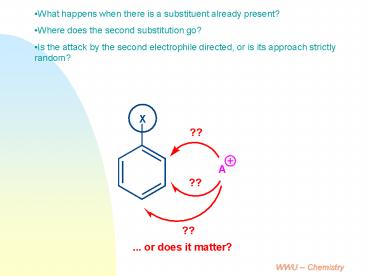
- What happens when there is a substituent already
present? - Where does the second substitution go?
- Is the attack by the second electrophile
directed, or is its approach strictly random?
2
In order to answer this kind of question, let us
examine two typical examples
3
The Nitration of Anisole
4
The Nitration of Anisole
- The first thing that we notice is that the
reaction is a great deal faster than the
nitration of benzene -- a sulfuric catalyst is
not required here. The methoxy group has
activated the ring toward electrophilic
substitution. - Second, the substitution is directed
substantially toward the para position with
respect to the methoxy group. - Some degree of substitution also seems directed
toward the ortho postion. - Subsitution in the meta position is negligible.
5
- In order to decide this question, let us consider
three alternative mechanisms - one to yield the ortho product
- one to yield the meta product
- one to yield the para product
- When we see the alternatives, perhaps we can
decide if one of them is better than the others! - In the following, assume that the preliminary
step to generate the electrophile (nitronium ion
is our example, here) has already happened - Also, assume that the last step (loss of proton
from the arenium ion to yield the final product)
will happen even though its not shown.
6
..
7
- The resonance stabilization of the arenium ion
for ortho substitution and for para substitution
is greater than for meta substitution. - We say, therefore, that the methoxy substituent
is an ortho-para director. - The ortho-para directing ability of the methoxy
group stems from its ability to delocalize its
unshared electron pairs in order to stabilize the
arenium ion -- this is an example of a resonance
effect. - The activating property of the methoxy group also
stems from its electron-releasing resonance
effect. - The electrophile sees a ring with enhanced
electron-density.
YUM!!!
8
But why is the para product major, while the
ortho product is minor?
9
The Nitration of Nitrobenzene
10
The Nitration of Nitrobenzene
- The first thing that we notice is that the
reaction is a great deal slower than the
nitration of benzene -- the sulfuric catalyst is
necessary here, and fuming nitric acid is also
required. The nitro group has deactivated the
ring toward electrophilic substitution. - Second, the substitution is directed
substantially toward the meta position with
respect to the nitro group. - Subsitution in the ortho and para positions is
negligible.
11
- In order to decide this question, let us again
consider three alternative mechanisms - one to yield the ortho product
- one to yield the meta product
- one to yield the para product
- When we see the alternatives, perhaps, once
again, we can decide if one of them is better
than the others! - As before, in the following, assume that the
preliminary step to generate the electrophile
(nitronium ion is our example, here) has already
happened - Also, assume that the last step (loss of proton
from the arenium ion to yield the final product)
will happen even though its not shown.
12
ortho
BAD!
meta
para
BAD!
13
- The resonance stabilization of the arenium ion
for ortho substitution and for para substitution
is worse than for meta substitution. - We say, therefore, that the nitro substituent is
a meta director. - The meta directing ability of the nitro group
stems from the positive charge borne by the atom
directly attached to the benzene ring. This
charge destabilizes resonance forms that place
positive charge on the adjacent ring carbon --
this is also an example of a resonance effect. - The deactivating property of the nitro group also
stems from its electron-withdrawing resonance
effect. - The electrophile sees a ring with diminished
electron-density.
YUCK!!!
14
Substituent Effects on Electrophilic Aromatic
Substitution
15
- In the following, note that
- -I represents an electron-withdrawing inductive
effect - I represents an electron-releasing inductive
effect - -R represents an electron-withdrawing resonance
effect - R represents an electron-releasing resonance
effect.
16
meta-Directing Substituents
17
- Why is -N(CH3)3 a -I group?
- Why isnt it also a -R group?
18
ortho-para-Directing Groups (Activating)
19
ortho-para-Directing Groups (Deactivating)
20
Where does the third substitution go, when there
are already two substituent groups on benzene?
21
- When there are two or more substituent groups
attached to benzene, they will compete. - If their directing effects complement one
another, it is easy to predict the position of
substitution. - If their directing effects do not complement one
another, then it is much more difficult to
predict where the next substitution will go. It
becomes necessary to compare the strengths of the
directing effects.
22
GROUPS ACTING IN CONCERT
steric crowding
o,p director
very little formed
m-director
HNO3
H2SO4
When groups direct to the same positions it is
easy to predict the product.
major product
23
GROUPS COMPETING
o,p-directing groups win over m-directing groups
too crowded
X
HNO3
H2SO4
24
RESONANCE EFFECT versus HYPERCONJUGATION
R
HNO3
H2SO4
major product
R (by hyperconjugation)
resonance effects are more important than
hyperconjugation
25
SOME GENERAL RULES
1) Activating (o,p) groups (R, I) win over
deactivating (m) groups (-R,-I).
2) Resonance groups (R) win over inductive
(I) groups or over groups that are R by
hyperconjugation.
3) 1,2,3-Trisubstituted products rarely form due
to excessive steric crowding.
4) With bulky directing groups, there will
usually be more p-substitution than
o-substitution.
5) The incoming group replaces a hydrogen, it
will not usually displace a substituent
already in place.
26
Predict where the aromatic substitution will take
place (to form the major product)
minor
27
More...