Buffer Solutions - PowerPoint PPT Presentation
1 / 49
Title:
Buffer Solutions
Description:
Methyl violet HInd H Ind- Acid Base. Indicators. Methyl violet HInd H Ind- Acid Base ... Methyl violet HInd H Ind- Acid Base. Yellow Blue. Shift Colour ... – PowerPoint PPT presentation
Number of Views:2013
Avg rating:3.0/5.0
Title: Buffer Solutions
1
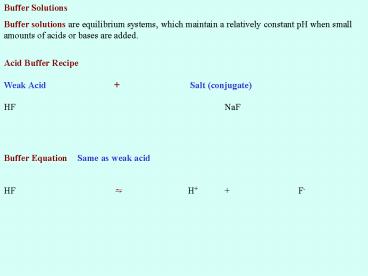
Buffer Solutions Buffer solutions are
equilibrium systems, which maintain a relatively
constant pH when small amounts of acids or bases
are added.
Acid Buffer Recipe Weak Acid Salt
(conjugate) HF NaF
Buffer Equation Same as weak acid HF ? H
F-
2
Buffer Solutions Buffer solutions are
equilibrium systems, which maintain a relatively
constant pH when small amounts of acids or bases
are added.
Acid Buffer Recipe Weak Acid Salt
(conjugate) HF NaF
Buffer Equation Same as weak acid HF ? H
F- High
3
Buffer Solutions Buffer solutions are
equilibrium systems, which maintain a relatively
constant pH when small amounts of acids or bases
are added.
Acid Buffer Recipe Weak Acid Salt
(conjugate) HF NaF
Buffer Equation Same as weak acid HF ? H
F- High low
4
Buffer Solutions Buffer solutions are
equilibrium systems, which maintain a relatively
constant pH when small amounts of acids or bases
are added.
Acid Buffer Recipe Weak Acid Salt
(conjugate) HF NaF
Buffer Equation Same as weak acid HF ? H
F- High low High
5
Buffer Equation Same as weak acid HF ? H
F- High low High
The HF is relatively high because HF is a weak
acid and does not dissociate very much. The H
is low for the same reason. The F- is high due
to adding the salt NaF to the solution. This
buffer solution has high concentrations of HF and
F-, which means it has the ability to shift left
or right and maintain the H concentration and
hence the pH remains at a relatively constant
level.
6
1.0 M HCN is mixed with 2.0 M NaCN to make a
buffer solution. Write the equation for the
buffer solution. Determine the HCN and
CN-. HCN ? H CN- 1.0 M
7
1.0 M HCN is mixed with 2.0 M NaCN to make a
buffer solution. Write the equation for the
buffer solution. Determine the HCN and
CN-. HCN ? H CN- 1.0 M Low
8
1.0 M HCN is mixed with 2.0 M NaCN to make a
buffer solution. Write the equation for the
buffer solution. Determine the HCN and
CN-. HCN ? H CN- 1.0 M Low 2.0 M
9
Base Buffer Recipe Weak Base Salt
(conjugate) NH3 NH4Cl
Buffer Equation Same as weak base NH3 H2O ? N
H4 OH-
10
Base Buffer Recipe Weak Base Salt
(conjugate) NH3 NH4Cl
Buffer Equation Same as weak base NH3 H2O ? N
H4 OH- High
11
Base Buffer Recipe Weak Base Salt
(conjugate) NH3 NH4Cl
Buffer Equation Same as weak base NH3 H2O ? N
H4 OH- High High
12
Base Buffer Recipe Weak Base Salt
(conjugate) NH3 NH4Cl
Buffer Equation Same as weak base NH3 H2O ? N
H4 OH- High High Low
13
Fill in the blanks to get buffer
solutions. Acid Base HCN
14
Fill in the blanks to get buffer
solutions. Acid Base HCN NaCN
15
Fill in the blanks to get buffer
solutions. Acid Base HCN NaCN N
aHCO3
16
Fill in the blanks to get buffer
solutions. Acid Base HCN NaCN H2CO3
NaHCO3
17
Fill in the blanks to get buffer
solutions. Acid Base HCN NaCN H2CO3
NaHCO3 H3PO4
18
Fill in the blanks to get buffer
solutions. Acid Base HCN NaCN H2CO3
NaHCO3 H3PO4 NaH2PO4
19
Fill in the blanks to get buffer
solutions. Acid Base HCN NaCN H2CO3
NaHCO3 H3PO4 NaH2PO4 KCH3COO
20
Fill in the blanks to get buffer
solutions. Acid Base HCN NaCN H2CO3
NaHCO3 H3PO4 NaH2PO4 CH3COOH KCH3CO
O
21
Fill in the blanks to get buffer
solutions. Acid Base HCN NaCN H2CO3
NaHCO3 H3PO4 NaH2PO4 CH3COOH KCH3CO
O NH4Cl
22
Fill in the blanks to get buffer
solutions. Acid Base HCN NaCN H2CO3
NaHCO3 H3PO4 NaH2PO4 CH3COOH KCH3CO
O NH4Cl NH3
23
Fill in the blanks to get buffer
solutions. Acid Base HCN NaCN H2CO3
NaHCO3 H3PO4 NaH2PO4 CH3COOH KCH3CO
O NH4Cl NH3 Na2CO3
24
Fill in the blanks to get buffer
solutions. Acid Base HCN NaCN H2CO3
NaHCO3 H3PO4 NaH2PO4 CH3COOH KCH3CO
O NH4Cl NH3 NaHCO3 Na2CO3
25
Fill in the blanks to get buffer
solutions. Acid Base HCN NaCN H2CO3
NaHCO3 H3PO4 NaH2PO4 CH3COOH KCH3CO
O NH4Cl NH3 NaHCO3 Na2CO3 NaH2PO4
26
Fill in the blanks to get buffer
solutions. Acid Base HCN NaCN H2CO3
NaHCO3 H3PO4 NaH2PO4 CH3COOH KCH3CO
O NH4Cl NH3 NaHCO3 Na2CO3 NaH2PO4
Na2HPO4
27
Buffer Application 1. Pick the two buffer
solutions A. NH4Cl and HCl B. NaCl and NaOH
C. HF and NaF D. NH4Cl and NH3
28
Buffer Application 1. Pick the two buffer
solutions A. NH4Cl and HCl B. NaCl and NaOH
C. HF and NaF D. NH4Cl and NH3
29
Buffer Application 1. Pick the two buffer
solutions A. NH4Cl and HCl B. NaCl and NaOH
C. HF and NaF D. NH4Cl and NH3 2. Write
the equations for the above two buffer solutions.
Label each species in the equation as high or
low concentration.
30
Buffer Application 1. Pick the two buffer
solutions A. NH4Cl and HCl B. NaCl and NaOH
C. HF and NaF D. NH4Cl and NH3 2. Write
the equations for the above two buffer solutions.
Label the each species in the equation as high
or low concentration.
HF ? H F- NH3 H2O NH4 OH-
31
Buffer Application 1. Pick the two buffer
solutions A. NH4Cl and HCl B. NaCl and NaOH
C. HF and NaF D. NH4Cl and NH3 2. Write
the equations for the above two buffer solutions.
Label the each species in the equation as high
or low concentration.
HF ? H F- High Low
High NH3 H2O NH4 OH-
32
Buffer Application 1. Pick the two buffer
solutions A. NH4Cl and HCl B. NaCl and NaOH
C. HF and NaF D. NH4Cl and NH3 2. Write
the equations for the above two buffer solutions.
Label the each species in the equation as high
or low concentration.
HF ? H F- High Low
High NH3 H2O NH4 OH- High
High Low
33
- A few drops of 0.10 M NaOH is added to a buffer
solution at pH 5.00. - The new pH could be
- A 4.98
- B. 5.02
- C. 8.05
- D. 2.11
34
- A few drops of 0.10 M NaOH is added to a buffer
solution at pH 5.00. - The new pH could be
- A 4.98
- B. 5.02
- C. 8.05
- D. 2.11
35
Indicators Methyl violet HInd ? H Ind-
Acid Base
36
Indicators Methyl violet HInd ? H Ind-
Acid Base Yellow Blue
37
Indicators Methyl violet HInd ? H Ind-
Acid Base Yellow Blue Shift Co
lour At pH lt 0.8
38
Indicators Methyl violet HInd ? H Ind-
Acid Base Yellow Blue Shift Co
lour At pH lt 0.8 Left
39
Indicators Methyl violet HInd ? H Ind-
Acid Base Yellow Blue Shift Co
lour At pH lt 0.8 Left Yellow
40
Indicators Methyl violet HInd ? H Ind-
Acid Base Yellow Blue Shift Co
lour At pH lt 0.8 Left Yellow HInd gt
Ind-
41
Indicators Methyl violet HInd ? H Ind-
Acid Base Yellow Blue Shift Co
lour At pH lt 0.8 Left Yellow HInd gt
Ind- At pH gt 0.8
42
Indicators Methyl violet HInd ? H Ind-
Acid Base Yellow Blue Shift Co
lour At pH lt 0.8 Left Yellow HInd gt
Ind- At pH gt 0.8 Right
43
Indicators Methyl violet HInd ? H Ind-
Acid Base Yellow Blue Shift Co
lour At pH lt 0.8 Left Yellow HInd gt
Ind- At pH gt 0.8 Right Blue
44
Indicators Methyl violet HInd ? H Ind-
Acid Base Yellow Blue Shift Co
lour At pH lt 0.8 Left Yellow HInd gt
Ind- At pH gt 0.8 Right Blue HInd lt
Ind-
45
Indicators Methyl violet HInd ? H Ind-
Acid Base Yellow Blue Shift Co
lour At pH lt 0.8 Left Yellow HInd gt
Ind- At pH gt 0.8 Right Blue HInd lt
Ind- At pH 0.8
46
Indicators Methyl violet HInd ? H Ind-
Acid Base Yellow Blue Shift Co
lour At pH lt 0.8 Left Yellow HInd gt
Ind- At pH gt 0.8 Right Blue HInd lt
Ind- At pH 0.8 Middle
47
Indicators Methyl violet HInd ? H Ind-
Acid Base Yellow Blue Shift Co
lour At pH lt 0.8 Left Yellow HInd gt
Ind- At pH gt 0.8 Right Blue HInd lt
Ind- At pH 0.8 Middle Green
48
Indicators Methyl violet HInd ? H Ind-
Acid Base Yellow Blue Shift Co
lour At pH lt 0.8 Left Yellow HInd gt
Ind- At pH gt 0.8 Right Blue HInd lt
Ind- At pH 0.8 Middle Green HInd
Ind-
49
_at_ the transition point Ka
H Ka 10-pH Ka 10-0.8 0.2