Halogenation reaction of alkane: a free radical substitution reaction' - PowerPoint PPT Presentation
1 / 10
Title:
Halogenation reaction of alkane: a free radical substitution reaction'
Description:
Halogenation reaction of alkane: a free radical substitution reaction. Alkenes react with halogen such as chlorine and bromine to produce haloalkanes ... – PowerPoint PPT presentation
Number of Views:2419
Avg rating:3.0/5.0
Title: Halogenation reaction of alkane: a free radical substitution reaction'
1
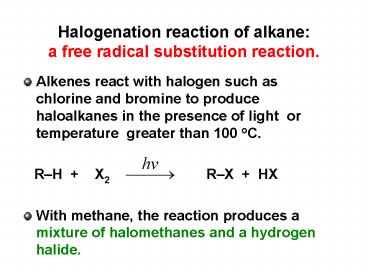
Halogenation reaction of alkane a free radical
substitution reaction.
- Alkenes react with halogen such as chlorine and
bromine to produce haloalkanes in the presence of
light or temperature greater than 100 oC. - RH X2 RX HX
- With methane, the reaction produces a mixture of
halomethanes and a hydrogen halide.
2
Examples
i. CH4 Cl2
CH3Cl CH2Cl2 CHCl3 CCl4 HCl
ii. CH3CH3 Cl2
CH3CH2Cl HCl
3
CH3
CH3
iii. CH3 CCH3 Cl2
CH3 CCH2Cl HCl
CH3
CH3
(ii) (iii) 1 product only because all the
hydrogen atoms are identical
4
CH3CH2CH2Br ( minor) CH3CHCH3
Br ( major) HBr
iv. CH3CH2CH3 Br2
5
Reaction mechanism
- CH4 Br2 CH3Br HBr
- Chain initiation step
Note illustrate electrons movement by using
half-headed curve arrow
VIDEO1
6
- Chain propagation steps
7
- Chain termination step.
8
Chain propagation step can be repeated to form
CH3Cl , CH2Cl2 , CHCl3 and CCl4 .
CH3 Cl ?? CH2Cl HCl
CH2Cl Cl2 ?? CH2Cl2 Cl
CH2Cl2 Cl ?? CHCl2 HCl
CHCl2 Cl2 ?? CHCl3 Cl
CHCl3 Cl ?? CCl3 HCl
CH3 Cl2 ?? CCl4 Cl
9
Example
- The bromination of 2-methylbutane(neopentane)
yields a mixture of isomers.
Increasing yield
10
Exercise
- Chlorination reaction of certain alkanes can be
used for laboratory preparations, for example in
the preparation of chlorocyclopentane from
cyclopentane. Give the mechanism for the reaction.












![Lecture note : Gas chromatography [2] ????????? ??? PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/6741367.th0.jpg?_=20150612015)

















