Lecture note : Gas chromatography [2] ????????? ??? - PowerPoint PPT Presentation
1 / 51
Title:
Lecture note : Gas chromatography [2] ????????? ???
Description:
A Photoionization Reaction If the amount of ionization is reproducible for a given compound, pressure, ... (like organosulfur or organophosphorus species) ... – PowerPoint PPT presentation
Number of Views:289
Avg rating:3.0/5.0
Title: Lecture note : Gas chromatography [2] ????????? ???
1
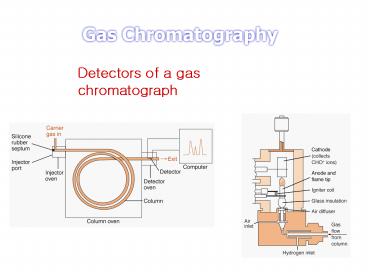
Detectors of a gas chromatograph
2
The Ideal Detector Adequate sensitivity - range
1018 to 1015 g analyte/s Good stability and
reproducibility A linear response to analyte
that extends over several orders of magnitude A
temperature range from room temperature to at
least 400o C A short response time that is
independent of flow rate High reliability and
ease of use Similarity in response toward all
analytes of alternatively a higher Predictable
and selective response toward classes of
analytes Nondestructive of sample http//www.pe
ople.virginia.edu/roa2s/chem_551/8/tsld002.htm
3
- Detector Requirements
- High Sensitivity
- Sensitivity refers to the change in
detector response as a function of the change in
the amount or concentration of the analyte. - S dR / dC
- or S dR / dQ
- where S is sensitivity, R is detector
response, C is concentration of the analyte in
the detector, and Q is the total quantity of the
analyte in the detector. - Detector sensitivity is best measured as
the slope of the calibration graph, a plot of
detector response vs. analyte concentration or
quantity.
4
The range over which the detector sensitivity is
constant is called the linear dynamic range, and
the entire range over which response varies with
concentration or quantity is called the dynamic
range of the detector. The lower limit of
detection is a function not only of the detector
sensitivity but of detector noise. Detector noise
is defined as the standard deviation of the
detector response when no sample is present and
is referred to as the root-mean-square noise
(Nrms). The detection limit (DL) is defined as
the quantity or concentration required to produce
a response which is three times the detector
noise). DL 3 Nrms / S H.H. Hill and
D.G. McMinn (Ed.), Detectors for Capillary
Chromatography, Wiley, 1992. pp 2-3.
5
2) High Selectivity The selectivity of a given
compound over a potentially interfering compound
can be measured by the ratio of the detector
sensitivities. Selectivity (SEL) is reported in
terms of relative molar response or as relative
weight response. SEL S1 / S2 Where S1
is the detector sensitivity of the compound of
interest and S2 is the detector sensitivity of
the potentially interfering compound. When
selectivity is greater than three orders of
magnitude for most potentially interfering
compounds, it is sometimes referred to as
specificity and the detector is said to be
specific for that compound or class of the
compounds.
6
Detector types are 1) FID ( flame ionization
detector ) 2) TCD ( thermal conductivity
detector ) 3) ECD ( electron capture detector
)4) FPD ( flame photometric detector ) 5) HID (
helium ionization detector ) 6) NPD (
nitrogen-phosphorus detector ) 7) PID ( photo
ionization detector ) 8) TID ( thermionic
ionization detector ) 9) CCD ( catalytic
combustion detector ) 10) NPD/DELCD ( combination
NPD and dry electrolytic conductivity detector )
11) FID/DELCD ( combination FID and dry
electrolytic conductivity detector ) 12) FID/FPD
( combination FID and FPD ) 13) Dual FPD ( dual
wavelength FPD for both sulfur and phosphorus )
14) FID dual FPD ( dual FPD plus FID combination
)
7
(No Transcript)
8
Thermal Conductivity Detector (TCD) Introduction
A TCD detector consists of an electrically-heated
wire or thermistor. The temperature of the
sensing element depends on the thermal
conductivity of the gas flowing around it.
Changes in thermal conductivity, such as when
organic molecules displace some of the carrier
gas, cause a temperature rise in the element
which is sensed as a change in resistance. The
TCD is not as sensitive as other detectors but it
is non-specific and non-destructive.
Instrumentation Two pairs of TCDs are used in
gas chromatographs. One pair is placed in the
column effluent to detect the separated
components as they leave the column, and another
pair is placed before the injector or in a
separate reference column. The resistances of the
two sets of pairs are then arranged in a bridge
circuit. The bridge circuit allows amplification
of resistance changes due to analytes passing
over the sample thermoconductors and does not
amplify changes in resistance that both sets of
detectors produce due to flow rate fluctuations,
etc.
9
Schematic of a bridge circuit for TCD
detection Two filament in one cell ( reference
side ) --- carrier gas only The other cell (
sample side ) --- carrier plus sample
flowing 1. Universal 2. Used primarily for gas
analysis 3. Sensitive few ppm
10
Flame Ionization Detector Introduction The
flame ionization detector (FID) is the most
sensitive gas chromatographic detector for
hydrocarbons such as butane or hexane. With a
linear range for 6 or 7 orders of magnitude (106
to 107) and limits of detection in the low
picogram or femtogram range, the FID is the gas
chromatographic detector for volatile
hydrocarbons and many carbon containing
compounds. FID Responds to all organic
compounds except for formic acid. Response
greatest with hydrocarbons and decreases with
substitution. Except for vapor of elements in
Groups I and II, does not respond to inorganic
compounds. Sensitivity high due to low noise
level. Insensitivity to water, the permanent
gases, and inorganic compounds simplifies the
resolution of components in analysis of aqueous
extracts and in air pollution studies.
11
Flame Ionization Detector Consists of a
stainless steel burner assembly installed in the
detector compartment and a electrometer system in
a separate unit adjacent to the gas chromatograph
Often it is installed in the tandem with the
thermal conductivity cell Effluent form the
column enters burner base through millipore
filters which remove contaminates Hydrogen mixed
with gas stream at bottom of jet and air or
oxygen is supplied axially around the jet.
Hydrogen flame burns at the tip, which also
functions as the cathode and is insulated form
the body by a ceramic seal Collector electrode
is above the burner tip and is made of platinum
12
An FID consists of a hydrogen/air flame and a
collector plate. The effluent from the GC column
passes through the flame, which breaks down
organic molecules and produces ions. The ions are
collected on a biased electrode and produce an
electrical signal. The FID is extremely sensitive
with a large dynamic range, its only disadvantage
is that it destroys the sample. FIDs are
normally heated independently of the
chromatographic oven. Heating is necessary in
order to prevent condensation of water generated
by the flame and also to prevent any hold-up of
solutes as they pass from the column to the
flame. With the flame extinguished, the column
end should be passed up through the jet and then
lightly held in position by slightly tightening
the coupling. Gradually draw the column end back
into detector jet until it is approximately 1 - 2
mm below the jet tip. Then tighten the coupling
to retain it in position. Do not over tighten
couplings on capillary columns.
13
Mechanism The effluent from the column is mixed
with hydrogen and air and then ignited
electrically at a small metal jet. Most organic
compounds produce ions and electrons that can
conduct electricity through the flame. There is
an electrode above the flame to collect the ions
formed at a hydrogen/air flame. The number of
ions hitting the collector is measured and a
signal is generated. In series with flame gases
is a selection of resistors 107 to 1010 ohms.
Vibrating reed electrometer used to provide
sensitivities up to 5 1013 Amps. Carbon
counting device that produces a current
proportional to number of ions or electrons
formed in the flamed gases. The organic molecules
undergo a series of reactions including thermal
fragmentation, chemi-ionization, ion molecule and
free radical reactions to produce
charged-species. The amount of ions produced is
roughly proportional to the number of reduced
carbon atoms present in the flame and hence the
number of molecules. Because the flame ionization
detector responds to the number of carbon atoms
entering the detector per unit of time, it is a
mass-sensitive, rather than a concentration-sensit
ive device. As a consequence, this detector has
the advantage that changes in flow rate of the
mobile phase has little effects on detector
response.
14
Normal Combustion i.e. burn methane in air and
get carbon dioxide and water vapor... CH4 O2 ?
CO2 H2O or CH4 3O2 ? CO2 2H2O Flame
Ionization during combustion, a uniform
proportion (about 0.0002) of the molecules in
this reaction do this instead (simplified for
clarity) CH4 O2 ? C H2O e- ? CO2
H2O or CH4 3O2 ? C O2 2H2O e- ? CO2
2H2O These oppositely-charged, intermediate
products can then be detected by the FID
15
Limitations Molecules that contained only
carbon and hydrogen respond best in this detector
but the presence of "heteroatoms" in a molecule,
such as oxygen, decreases the detector's
response. For instance, the FID's methane
response (CH4) is fabulous but formaldehyde's
(CH2O) is quite poor. Therefore, highly
oxygenated molecules or sulfides might best be
detected using another detector instead of the
FID. Sulfides determination by the flame
photometric detector and aldehydes and ketones
analyzed with the photoionization detector are
alternatives to the use of the FID for those
molecules.
16
Functional group, such as carbonyl, alcohol,
halogen, and amine, yield fewer ions or none at
all in a flame. In addition, the detector is
insensitive toward noncombustible gases such as
H2O, CO2, SO2 and NOx. Selectivity Compounds
with C-H bonds. A poor response for some
non-hydrogen containing organics (e.g.,
hexachlorobenzene). Sensitivity 0.1 10
ng Linear range 105 107 Gases Combustion
- hydrogen and air Makeup - helium
or nitrogen Temperature 250-300 C 400-450 C
for high temperature analyses
17
Detector Construction FID is constructed of a
small volume chamber into which the gas
chromatograph's capillary column in directly
plumbed. Usually the small diameter capillary
is fitted directly into the bottom of the
detector's flame jet. The gaseous eluents from
the column are mixed with separately plumbed in
hydrogen and air and all are burned on the jet's
tip. After the fuel (H2) and oxidant (O2 in air)
are begun, the flame is lit using a electronic
ignite, actually an electrically heated filament
that is turned on only to light the flame. The
charged particles created in that combustion
process create a current between the detector's
electrodes. One electrode is actually the
metallic jet itself, another is close by and
above the jet. The gaseous products leave the
detector chamber via the exhaust. The detector
housing is heated so that gases produced by the
combustion (mainly water) do not condense in the
detector before leaving the detector chimney.
18
View of TCD and FID of HP5890 GC
Flame Ionization Detector
19
Makeup Gases The total volume of gas in the FID
that yields the most sensitive and widest linear
response is not the same volume of gas when the
column effluent flow ( 1 mL/min) and hydrogen
and air flows are flowing these gases' total
flow into the detector is too small. Another way
to say this is that the optimum column flow to
maintain the best chromatography and the best
fuel and oxidant flows for the best flame
conditions--all added together--don't create the
best gas flow for the FID detector's design. This
means that to maintain the best analytical
conditions, additional gas must be constantly
flowed into the detector. This gas makes up the
additional needed gas flow and so is termed
makeup gas. Since the gas needs to be inert so
that its addition doesn't upset the fuel and
oxidant balance and since it needs to be added in
relatively large amounts (30 ml/min in some
detector designs) nitrogen is usually the gas of
choice. Helium would work also but is a
nonrenewable resource and more expensive. All gas
flows are controlled by adjustable gas
regulators.
20
Electron Capture Detector (ECD) The ECD uses a
radioactive ? emitter (electrons) to ionize some
of the carrier gas and produce a current between
a biased pair of electrodes. When organic
molecules that contain electronegative functional
groups, such as halogens, phosphorous, and nitro
groups pass by the detector, they capture some of
the electrons and reduce the current measured
between the electrodes. The ECD is as sensitive
as the FID but has a limited dynamic range and
finds its greatest application in analysis of
halogenated compounds.
Schematic of an ECD
21
ECD Selective in its response and highly
sensitive Hewlett Packard makes one with a
detection limit of less than 8 fg/sec for lindane
Sensitive toward molecules with electronegative
functional groups (halogens, peroxides, quinones,
nitro groups) Insensitive towards amines,
alcohols and hydrocarbons A leak test of an ECD
containing nickel-63 (63Ni) must be performed at
intervals not to exceed six months. The test
must be performed in accordance with the
manufacturer's instructions, or by wiping the gas
intake and outlet surfaces. NOTE Never attempt
to directly wipe the inner surface of the
component containing the radioactive material.
This might cause the ECD to fail and will
contaminate the ECD, the gas chromatograph and
the surrounding area. Never open the detector
cell for any reason.
22
Nitrogen Phosphorous Detector Specific sample
must contain nitrogen or phosphorous
Destructive LOD 0.4 pg N / sec 0.2 pg P /
sec Linear range 104 Mode of operation
essentially a modified FID
Active element acts to block undesired
species
23
Flame Photometric Detector The determination of
sulfur or phosphorus containing compounds is the
job of the flame photometric detector (FPD). This
device uses the chemiluminescent reactions of
these compounds in a hydrogen/air flame as a
source of analytical information that is
relatively specific for substances containing
these two kinds of atoms. The emitting species
for sulfur compounds is excited S2. The lambda
max for emission of excited S2 is approximately
394 nm. The emitter for phosphorus compounds in
the flame is excited HPO (lambda max doublet
510-526 nm). In order to selectively detect one
or the other family of compounds as it elutes
from the GC column, an interference filter is
used between the flame and the photomultiplier
tube (PMT) to isolate the appropriate emission
band. The drawback here being that the filter
must be exchanged between chromatographic runs if
the other family of compounds is to be detected.
Instrumentation In addition to the instrumental
requirements for 1) a combustion chamber to house
the flame, 2) gas lines for hydrogen (fuel) and
air (oxidant), and 3) an exhaust chimney to
remove combustion products, the final component
necessary for this instrument is a thermal
(bandpass) filter to isolate only the visible and
UV radiation emitted by the flame. Without this
the large amounts of infrared radiation emitted
by the flame's combustion reaction would heat up
the PMT and increase its background signal. The
PMT is also physically insulated from the
combustion chamber by using poorly (thermally)
conducting metals to attach the PMT housing,
filters, etc. The physical arrangement of these
components is as follows flame (combustion)
chamber with exhaust, permanent thermal filter
(two IR filters in some commercial designs), a
removable phosphorus or sulfur selective filter,
and finally the PMT.
24
Schematic of a gas chromatographic flame
photometric detector Specific phosphorous or
sulfur Destructive LOD 20 pg S /sec, 0.9 pg P /
sec Linbear range 104 P, 103 S
25
Photoionization Detector Introduction The reason
to use more than one kind of detector for gas
chromatography is to achieve selective and/or
highly sensitive detection of specific compounds
encountered in particular chromatographic
analyses. The selective determination of aromatic
hydrocarbons or organo-heteroatom species is the
job of the photoionization detector (PID). This
device uses ultraviolet light as a means of
ionizing an analyte exiting from a GC column. The
ions produced by this process are collected by
electrodes. The current generated is therefore a
measure of the analyte concentration. Theory If
the energy of an incoming photon is high enough
(and the molecule is quantum mechanically
"allowed" to absorb the photon) photo-excitation
can occur to such an extent that an electron is
completely removed from its molecular orbital,
i.e. ionization. A Photoionization Reaction
26
If the amount of ionization is reproducible for a
given compound, pressure, and light source then
the current collected at the PID's reaction cell
electrodes is reproducibly proportional to the
amount of that compound entering the cell. The
reason why the compounds that are routinely
analyzed are either aromatic hydrocarbons or
heteroatom containing compounds (like
organosulfur or organophosphorus species) is
because these species have ionization potentials
(IP) that are within reach of commercially
available UV lamps. The available lamp energies
range from 8.3 to 11.7 ev, that is, lambda max
ranging from 150 nm to 106 nm. Although most PIDs
have only one lamp, lamps in the PID are
exchanged depending on the compound selectivity
required in the analysis.
27
Selective detection using a PID Here is an
example of selective PID detection Benzene's
boiling point is 80.1 degrees C and its IP is
9.24 ev. (Check the CRC Handbook 56th ed. page
E-74 for IPs of common molecules.) This compound
would respond in a PID with a UV lamp of 9.5 ev
(commercially available) because this energy is
higher than benzene's IP (9.24). Isopropyl
alcohol has a similar boiling point (82.5 degrees
C) and these two compounds might elute relatively
close together in normal temperature programmed
gas chromatography, especially if a fast
temperature ramp were used. However, since
isopropyl alcohol's IP is 10.15 ev this compound
would be invisible or show very poor response in
that PID, and therefore the detector would
respond to one compound but not the other.
28
Instrumentation Since only a small (but basically
unknown) fraction of the analyte molecules are
actually ionized in the PID chamber, this is
considered to be a nondestructive GC detector.
Therefore, the exhaust port of the PID can be
connected to another detector in series with the
PID. In this way data from two different
detectors can be taken simultaneously, and
selective detection of PID responsive compounds
augmented by response from, say, an FID or ECD.
The major challenge here is to make the design of
the ionization chamber and the downstream
connections to the second detector as low volume
as possible (read small diameter) so that peaks
that have been separated by the GC column do not
broaden out before detection.
Specific compounds ionized by UV LOD 2 pg
Carbon / sec Linear range 107
29
Atomic-Emission Detector (AED) This detector,
while quite expensive compared to other
commercially available GC detectors, is an
extremely powerful alternative. For instance,
Instead of measuring simple gas phase (carbon
containing) ions created in a flame as with the
flame ionization detector, or the change in
background current because of electronegative
element capture of thermal electrons as with the
electron capture detector, the AED has a much
wider applicability because it is based on the
detection of atomic emissions. The strength of
the AED lies in the detector's ability to
simultaneously determine the atomic emissions of
many of the elements in analytes that elute from
a GC capillary column (called eluants or solutes
in some books). As eluants come off the capillary
column they are fed into a microwave powered
plasma (or discharge) cavity where the compounds
are destroyed and their atoms are excited by the
energy of the plasma. The light that is emitted
by the excited particles is separated into
individual lines via a photodiode array. The
associated computer then sorts out the individual
emission lines and can produce chromatograms made
up of peaks from eluants that contain only a
specific element.
30
Instrumentation The components of the AED
include 1) an interface for the incoming
capillary GC column to the microwave induced
plasma chamber, 2) the microwave chamber itself,
3) a cooling system for that chamber, 4) a
diffraction grating and associated optics to
focus then disperse the spectral atomic lines,
and 5) a position adjustable photodiode array
interfaced to a computer. The microwave cavity
cooling is required because much of the energy
focused into the cavity is converted to heat.
Schematic of a gas chromatographic atomic
emission detector
31
GC Analysis Qualitative --- determine what is
present 1)
Chromatographic a) tR or
Retention Index b) Spiking
2) Spectroscopic a) Sample
collection --- MS, IR b)
Dynamic GC/MS c) IR, GC/FTIR
spectrometer d) NMR
Quantitative -- determine how much is present
use peak height h or area A
32
Qualitative analysis tR Standard ----
methanol, MEK(tR ), toluene Unknown -----
same tR (X) Conclude (X) MEK
Retention time limitations tR changes with flow
rate, column temperature, liquid phase, column
history, sample size
WARNING identical retention times do not
confirm peak identity
33
Spiking Step 1 Peak X --- toluene ?
Step 2 Toluene added to sample Step 3
Peak X identified as toluene
34
Kovat Retention Index Isothermal I 100n
100(log tR(x) log tR(n)) / (log tR(n1)-log
tR(n)) I retention index x substance of
interest n n-alkane with n carbon atoms
emerging before the substance of interest
n1 n-alkane with n1 carbon atoms emerging
after the substance of interest.
Temperature programming I 100n
100(TR(x) TR(n))/(TR(n1)-TR(n))
TR elution temperature (K)
35
Kovat retention index All that is really being
done is to normalize each component compared to
n-alkanes. It assumes that you are dealing with
either identical or at least very similar columns
or paackings. Packing that have large differences
can result in peaks eluting in different
orders--- the method would then be useless.
36
(No Transcript)
37
Identification --- trapping 1) GC detector ---
melting point capillary --- sample condensate 2)
GC detector --- cold solvent trap --- glass wool
plug sample
condensate
ON-LINE and OFF-LINE system 1) OFF-LINE
- Fraction trapped and later analyzed -
Cumbersome, prome to contamination 2) ON-LINE
- Fraction analyzed in real time as they
elute - Requires high speed spectrometer
for small samples, low concentrations
38
Quantitative GC procedure 1) Sampling
2) Sample preparation 3) Chromatography
4) Integration 5) Calculation ---- a)
Simple normalization
b) Corrected area normalization
c) External standard
d) Internal
standard e)
Standard addition
39
Accuracy --- Goal of analysis 1) Absolute
error difference between measured and true
2) Relative () error error / true
value x 100 ex. True value
50 g Measured
48 g Absolute error 2 g
error ( 2 g / 50 g ) x
100 4 Precision 1) Measures
reproducibility 2) Measures techniques
Average SD
RSD Importance of
RSD Precision
Calibration Accuracy
40
Sampling Objective ------ take small sample
representative of larger population Possible
errors -- 1) Non-representative
2) Contamination Sample
preparation 1) Crush 2) Dissolve
3) Filter 4) Extract 5) Dilute 6)
Concentrate 7) Derivatize Possible
error --- sample loss, change, contamination Chro
matography Possible error --- loss sample,
leaks,
non eluting,
overlapping or undetected
peaks detector,
recorder problems
41
Digital conversion --- peak height 1)
Advantage -------- easy, rapid, inexpensive
2) Possible errors --- peaks unresolved,
too small,
off scale,
drifting
baseline Integration --- peak area 1)
manual methods Possible errors --- peaks
unresolved, too small, off scale, drifting
baseline 2) Integration --- mechanical
3) Integration --- Digital electronic
4) Computing integrators
Hewlett Packard - Model 3396A Integrator
42
Simple normalization Peak A
B C Area 150 300
600 Weight A ( area A / total area ) x
100 150 / ( 150
300 600) x 100
14.3 Assumes 1) A B C 100
2) Detector shows equal response
for A,B, and C Response factor (RF)
Peaks A B C Weight
10 10 10 microgram Area
150 300 600 Response
factors not equal 1) Simple area
normalization not valid 2) Must
calculate RF
43
Calculation of response factors
ex. Slope ?A / ?W 90 / 3 30
Corrected area normalization Peak
Area RF Corrected area A
150 15 10 B
300 30 10 C
600 60 10
--------------------------------------------------
---
Total 30 Weight A ( Corrected area A /
Total corrected areas) x 100 (10 / 30) 100
33.3 Method still
assumes A B C 100
44
External standard method
1) Make calibration curve Area vs
Weight(microgram) 2) Inject known weight of
sample 3) Measure area read weight of
component from calibration curve 4) Weight
weight unknown component x 100
weight sample Must know exact volume of
injection Best to use sample value
Area
weight unknown component
Weight(microgram)
45
Internal standard method 1st step --- choose
IS 1) Never found in sample peak 2)
Well resolved 3) Add to sample at
concentration of analyte having
similar response 4) Available pure 2nd
step --- calibration 1) Prepare standard
mixtures 2) Chromatograph standards
3) Plot area ratio vs weight ratio
AX / AIS
WX / WIS
46
3rd step --- analyze sample 1) Mix IS with
sample X weights known accurately 2)
Chromatograph mixture 3) Measure areas
calculate ratio 4) Interpolate curve to
give weight ratio for X 5) (WX / WIS) x
WIS WX for X
AX / AIS
WX / WIS
47
Standard addition method
Area
4 2 2 4 6 8
Weight of analyte
added( microgram ) Weight of X
3.2 microgram Especially useful for
dirty samples Interferences same for standards
and unknown
48
Specific applications of GC 1. Analysis of
ketones, aldehydes, aromatics,.... 2. Analysis
of steroids. 3. Analysis of pesticides. 4.
Analysis of blood components. 5. Analysis of
old, petroleum and petroleum products. 6.
Environmental(air and water) pollution VOC,
PAH etc. 7. Foods. 8. Pharmaceuticals. 9.
Anything that can be volatilized and pushed
through a column.
49
(No Transcript)
50
High speed chromatogram obtained with isothermal
operation (30oC) for 37 sec followed by a
35oC/min temperature ramp to 90oC.
51
Typical gas solid chromatogram on a PLOT




![Lecture note : Gas chromatography [1] ????????? PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/6692254.th0.jpg?_=20150604093)
![Lecture note : Gas chromatography [1] ????????? PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/4420183.th0.jpg?_=20131029062)
![Lecture note : Gas chromatography [2] ????????? ??? PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/4668905.th0.jpg?_=20131120112)























