Functional Group
1 / 85
Title: Functional Group
1
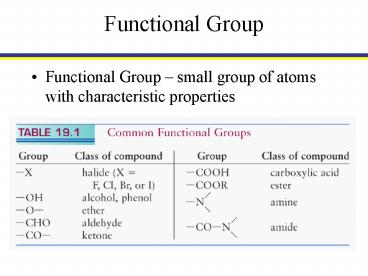
Functional Group
- Functional Group small group of atoms with
characteristic properties
2
Haloalkanes
- At least one halogen atom has replaced a hydrogen
atom on the hydrocarbon chain - Nomenclature for haloalkanes
- Name the halogen as a substituent.
- fluoro, chloro, bromo, iodo
CH3CH2C(CH3)ClCH2CHClCH3
CH3CH2CCCBr3
3
Halogens on Aromatics
1,2-dichlorobenzene
o-dichlorobenzene
1,4-diiodobenzene
chlorobenzene
1-chloro-1-phenylethane
p-diiodobenzene
4
Properties and Uses of Haloalkanes
CFC
Pesticides Refrigerants High toxicity
Lindane
DDT 1,1,1-trichloro-2,2-bis-p-chlorophenylethane
Chlordan
5
Reactivity of Haloalkanes
Cl
Polar compound because the electron withdrawing
strength of the halogen
Nucleophilic substitution
6
Nucleophilic Substitution
- A reaction in which a nucleophile replaces the
halogen on the molecule - nucleophile
- a reactant attracted
- to centers of
- positive charge
N- R X ? N R X-
If N- OH- ? alcohol formation
OR- ? ether formation NH2- ?
amine formation RCOO- ? ester
formation CN- ? nitrile formation
SH- ? mercaptan formation
7
Nucleophilic Substitution Mechanisms
- SN1 reaction
- S substitution
- N nucleophilic
- 1 molecularity
- Formation of carbocation
- Following by nucleophile attack
- Forms racemic mixture if molecule is chiral
- SN2 reaction
- S substitution
- N nucleophilic
- 2 molecularity
- Simultaneous nucleophile attack while leaving
group leaves molecule - Complex is formed
- Inversion of molecule results in formation of
opposite enantiomer (if molecule is chiral)
8
SN1 reaction (heterolytic dissociation)
Rate determining step is unimolecular
I-
?
carbocation
?
9
SN1 reaction (heterolytic dissociation)
?
H3O
10
SN2 reaction
Entering group
Leaving group
Rate determining step is unimolecular
?
Complex
?
I-
Inversion of product
11
SN1 or SN2?
Why does one of these molecules undergo
substitution by SN1 while the other undergoes
SN2?
Steric hinderance
12
Example Problem
- A pure, optically active sample of one isomer of
CHCl(C6H5)CH3 is hydrolyzed by water, and the
product is optically inactive. - Is the mechanism SN1 or SN2?
- Write the formula of the product.
13
Example Problem
- The following haloalkane compounds undergo
nucleophilic substitution by the SN1 mechanism.
Which of the following would you expect to
proceed more rapidly - a) CH3F b) CH3Cl c) CH3Br d) CH3I
14
Nomenclature for Functional Groups
If N- OH- ? alcohol formation
OR- ? ether formation NH2- ?
amine formation RCOO- ? ester
formation CN- ? nitrile formation
SH- ? mercaptan formation
15
Alcohols
ethanol
methanol
2-propanol
1-propanol
16
Alcohols
2-methyl-2-propanol
Tertiary alcohol three carbon chains attached
to carbon with OH functional group
17
Phenol
phenol
hydroxybenzene
18
Physical Properties of Alcohols
- Boiling Pts of alkanes
- CH4 -162 oC
- C2H6 -88.5 oC
- C3H8 -42 oC
- C4H10 0 oC
- Boiling pts of alcohols
- CH3OH 64.5 oC
- C2H5OH 78.3 oC
- C3H7OH 97.0 oC
- C4H9OH 188 oC
1) BP of alcohols are higher due to hydrogen
bonding 2) BP of molecules increase with
increasing molar mass this results because of
increased London forces
19
Ethers
dimethyl ether
ethylmethyl ether
diethyl ether
2-ethoxyethanol
Can also call an O-R alkoxy group O-C2H5 would
be ethoxy
20
Physical Properties of Ethers
- Boiling pts of alcohols
- CH3OH 64.5 oC
- C2H5OH 78.3 oC
- C3H7OH 97.0 oC
- C4H9OH 188 oC
- Boiling pts of ethers
- CH3-O-CH3 -24 oC
- C2H5-O-C2H5 34.6 oC
1) BP of alcohols are higher due to hydrogen
bonding
21
Amines
methanamine
Ethanamine or aminoethane
aminomethane
dimethylamine (secondary)
trimethylamine (tertiary)
22
Physical Properties of Amines
- Boiling pts of alcohols
- CH3OH 64.5 oC
- C2H5OH 78.3 oC
- C3H7OH 97.0 oC
- C4H9OH 188 oC
- Boiling pts of amines
- CH3NH2 -7.5 oC
- C2H5NH2 17 oC
- C3H7NH2 49 oC
- C4H9NH2 78 oC
Amines are generally basic
ethers CH3-O-CH3 -24 oC C2H5-O-C2H5
34.6 oC
Amines have hydrogen bonding not as strong as
alcohols. Ethers do not have hydrogen bonding.
23
Functional Groups
-ol
-al
-amine
-amide
-oic acid
-oate
-one
24
Esters
methyl ethanoate or methyl acetate
methyl methanoate or methyl formate
ethyl ethanoate or ethyl acetate
25
Properties of Esters
- Fragrant odors
- Naturally occurring as fats and oils
26
Alkene Preparation
- Elimination Reaction
- Dehydrohalogenation removing a hydrogen and
halogen atom from the molecule - CH3CH2CHBrCH3 ? CH3CH CHCH3
27
Elimination Reaction
2-butene
1-butene
?
28
Elimination Reaction
- Dehydration starting with an alcohol, eliminate
water and produce an alkene - Dehydrogenation removing two hydrogen atoms
from the molecule (difficult) - CH3CH3 ? CH2 CH2
- Requires a catalyst in which a complex surface
reaction must occur
29
Electrophilic Addition Reactions
Alkenes and alkynes are more reactive than alkanes
- Alkenes (and alkynes) contain double bonds due to
the formation of p bonds - p bonds are electron rich regions
What will be attracted to the negative p region?
Electrophile a reactant attracted to the region
of high electron density
30
Electrophilic Addition Reactions
Br -
?
Br -
31
Types of Addition Reactions
- Hydrogenation H2
- Halogenation Br2, Cl2
- Hydrohalogenation HBr, HCl
- Hydration HOH
32
Aromatic Reactions
- Predominant reaction electrophilic substitution
- Benzene rings have a highly dense electron region
Special stability of the benzene ring does not
favor addition reactions aromatics are generally
less reactive than alkenes
33
Mechanism for Electrophilic Substitution
Nitration of benzene
HNO3 H2SO4 ? NO2 HSO4- H2O
34
Adding a
Second Substituent
Electron withdrawing substituents are meta
directors
The presence of the first substituent will impact
the environment of the overall structure. How?
Example meta directors NO2, COOH, CN, CHO, SO3H
35
Adding a
Second Substituent
Electron donating substituents are ortho/para
directors
If phenol were nitrated (NO2), what would the
major product(s) be?
Example o/p directors OH, NH2, Cl, Br,
2 main products are o and p
36
Types of Polymerization
Polymer macromolecule made up of a great many
smaller repeating units
- Addition polymerization
- Chain-reaction polymerization
- Freeradical chain reaction polymerization
- Condensation polymerization
- Step-reaction polymerization
- Copolymerization
- Composite material
37
Addition polymerization
2
38
Plastics
Monomer The repeating unit of a polymer.
39
Recycling Plastics
http//www.obviously.
com/recycle/
- Type 1 recycled soda water containers
- Type 2 recycled milk, detergent, oil
bottles - Type 3 not commonly recycled PVC
- Type 4 not commonly recycled plastic bags,
shrink wrap - Type 5 not commonly recycled bottle tops,
carpets, containers - Type 6 not recycled throwaway utensils,
protective packing - Type 7 not recycled layered or mixed plastics
40
Electrically Conducting Plastics
Electrons move from p bond to p bond
Adding I3- ion to structure enhances the
conductivity
41
Electrically Conducting Plastics
Dupont calls this Olight technology
IBM calls this OLED technology
42
Flat-Screen Technology
- High brightness and contrast
- Ultra-wide viewing angle
- No backlight required
- Thin, compact form factor
- Fast response time
- Low power consumption
43
Condensation Polymerization
- Polyester
- Polyamide
- Combines a carboxylic acid with an amine
- nylon
Alcohol
H2O
Carboxylic acid
44
Copolymers
Polymers made up by more than one type of monomer
45
Composite
- Consists of two or more materials that have been
solidified together. - Seashells
- Bones
- Silicon carbide ceramic embedded with silicon
carbide fibers - The fibers are made from the polymer
dimethylsilane - Ceramics are brittle, but embedding the polymer
enhances structural integrity
46
Physical Properties of Polymers
- No definite masses chain-lengths
- No definite melting point gradually soften, high
viscosity - Strength depends on intermolecular forces if
substituent groups can form H-bonds, get stronger
forces if the chain is longer, get stronger
London forces either results in a stronger
polymer - Elasticity ability to return to original state
47
Aldehydes, Ketones,
and Carboxylic Acids
- All contain the carbonyl group (C O)
Carboxylic acid
Ketone
Aldehyde
48
Aldehydes, Ketones,
and Carboxylic Acids
2-butanone
propanal
propanioc acid
49
Oxidation of Alcohols
Primary alcohol
O2 (g) ?
Alcohols can be oxidized into aldehydes, which
can be further oxidized into carboxylic acids
O2 (g) ?
50
Oxidation of Alcohols
Secondary alcohol
O2 (g) ?
Can a ketone be further oxidized to produce a
carboxylic acid?
51
Intermolecular Interactions
- Why is the solubility, FP, and BP increased?
- Changes in intermolecular interactions
- Alkanes, alkenes, alkynes have low solubility (in
water), low FP and BP - London dispersion forces
- Carbonyl compounds have higher solubility (in
water), higher FP and BP - Dipole dipole interactions
52
Solvents
- Increased solubility in water makes these
substances good solvents. - O
- Acetone (propanone) CH3 C CH3
- Completely miscible in water, yet dissolves many
organic substances
53
Carboxylic Acids
Propanal 49 oC Propanoic acid 141 oC
- Hydrogen bonding
- Carboxyl group
- O
- R C OH
- Hydrogen bonding results in high solubilities, FP
and BP
54
Carboxylic Acids
- Weak acids O
- HC2H3O2 acetic acid CH3 - C O H
- Vinegar, very important base product for polymer
production - rayon, cellophane
- Acetylsalicylic acid ?
55
Organic Bases
- Amines hydrocarbon group with N attached
- Will amines be soluble?
secondary
tertiary
primary
N H bond in the amine results in hydrogen
bonding between molecules these compounds will
be soluble in water when the molecules are small
56
Amides
- Formed through a condensation reaction of a
carboxylic acid and amine (acid/base reaction) - Salt is an amide
acid
base
salt
57
Amide Linkage
- Amide linkage resulting
- from the condensation
- reaction is very important
- biologically.
- When the carboxylic acid and amine compounds are
both amino acids, the resulting amide linkage is
a peptide bond.
58
Example Amides
LSD
Acetaminophen
59
Amide Linkage
- When the amide linkage
- results from the bonding
- of two amino acids, the bond
- is called a peptide bond.
60
(No Transcript)
61
(No Transcript)
62
Peptide Bond
- Bond between two amino acids
dipeptide
a carbon
63
Proteins
- Long chain of amino acids bound through repeating
a-carbon, amide linkage, a-carbon (50 to several
thousand) - Many possible sequences of amino acids
SEQUENCE ? SHAPE ? FUNCTION
64
Amino acid sequence
- Primary structure determines the proteins
unique identity - Three fragments of human hemoglobin
- Leu-Ser-Pro-Ala-Asp-Lys-Thr-Asn-Val-Lys-
- -Val-Lys-Gly-Trp-Ala-Ala-
- -Ser-Thr-Val-Leu-Thr-Ser-Lys-Ser-Lys-Tyr-Arg
65
Shape
Hydrogen bonding
a-helix
- Secondary structure
- the arrangement of the
- chain in a regular pattern.
- Dependent on amino
- acid sequence
66
Secondary Structure
Triple helix
b-sheet
67
Function
- Tertiary structure overall shape
- Globular
- Fibrous
- di-sulfide bonds are examples of forces that help
to fold proteins into tertiary structures - Enzymes tertiary structure determines what
other substances can bind to the enzyme
68
Quaternary Structure
- Specific arrangement of neighboring polypeptide
units - Denaturation loss of one or any of the four
structures
69
4 Levels of Protein Structure
70
Carbohydrates
- Monosaccharides
71
(No Transcript)
72
Aldopentose
73
Aldohexose
74
(No Transcript)
75
Ketopentose
76
Ketohexose
77
Forms of Fructose
- ketose ketone sugar
- polyhydroxy ketone
78
Forms of Glucose
- Aldose aldehyde sugar
- polyhydroxy aldehyde
79
(No Transcript)
80
Condensation Reactions
- Disaccharides
- Maltose, lactose, cellobiose, sucrose
- Polysaccharides
- Starch (Glycogen)
- Formed through linkages of the a form
- Cellulose
- Formed through linkages of the b form
81
starch
cellulose
82
Nucleic Acids
- DNA deoxyribonucleic acid
- RNA ribonucleic acid
- Nucleotide monomer unit of nucleic acid
- Phosphoric acid molecule
- Five-carbon sugar
- Nitrogen-containing organic base
83
Nucleotides
84
(No Transcript)
85
Complementary base pairs