The Periodic Table Ch. 6 - PowerPoint PPT Presentation
Title:
The Periodic Table Ch. 6
Description:
The Periodic Table Ch. 6 Why is the Periodic Table so important to chemists? Three Classes of Elements Groups Elements in the same group have similar chemical and ... – PowerPoint PPT presentation
Number of Views:241
Avg rating:3.0/5.0
Title: The Periodic Table Ch. 6
1
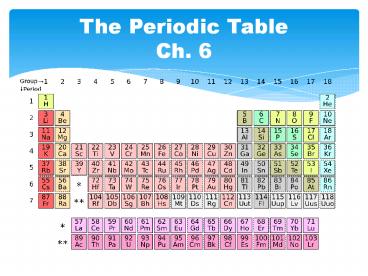
The Periodic TableCh. 6
2
Why is the Periodic Table so important to
chemists?
3
Three Classes of Elements
4
Groups
- Elements in the same group have similar chemical
and physical properties
- Why??
5
Families
- Columns are also grouped into families
- Families may be one column or several
- Families have names rather than numbers
6
Hydrogen
- A family of its own
- Diatomic
- Reactive gas
7
Alkali Metals
- Group 1A (No hydrogen)
- Very reactive metals
- Always combined with something else in nature
- Soft metals
8
Alkaline Earth Metals
- Group 2A
- Reactive metals
- Always combined
- with nonmetals in nature
- Some are important mineral nutrients (Mg and Ca)
9
Transition Metals
- d-Block elements
- Less reactive harder metals
- Includes metals used in jewelry and construction
- Cannot easily predict valence e-
10
Carbon Family
- Elements in group 4A
- Branch of chemistry based on Carbon
- Silicon and Germanium are important semiconductors
11
Halogens
- Group 7A
- Reactive
- Volatile
- Diatomic (F2,Cl2,Br2,I2)
- Nonmetals
- Always found combined with other elements in
nature
12
The Noble Gases
- Group 8A
- Unreactive gases (Inert)
- Have a full valence shell
13
Inner Transition Metals
- -Have f orbitals that contain electrons
- -Used to be called rare-earth elements
- -More abundant than other metals
14
Representative Elements
- Groups 1A 7A