Using the Periodic Table - PowerPoint PPT Presentation
1 / 11
Title:
Using the Periodic Table
Description:
Using the Periodic Table The boxes that make up the periodic table contain a significant amount of information. To understand this information, it is necessary to ... – PowerPoint PPT presentation
Number of Views:165
Avg rating:3.0/5.0
Title: Using the Periodic Table
1
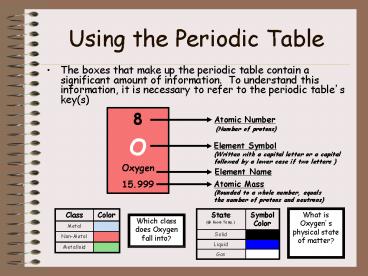
Using the Periodic Table
- The boxes that make up the periodic table contain
a significant amount of information. To
understand this information, it is necessary to
refer to the periodic tables key(s)
8 O Oxygen 15.999
Atomic Number (Number of protons)
Element Symbol (Written with a capital letter or
a capital followed by a lower case if two letters
)
Element Name
Atomic Mass (Rounded to a whole number, equals
the number of protons and neutrons)
Class Color
Metal
Non-Metal
Metalloid
State (_at_ Room Temp.) Symbol Color
Solid
Liquid
Gas
What is Oxygens physical state of matter?
Which class does Oxygen fall into?
2
The Periodic Table of Elements
3
Element
- A pure substance made up of one kind of atom that
cannot be broken down into simpler substances by
physical or chemical means - 90 occur naturally on earth
- 25 were synthesized (made) by scientists
Element Song
http//www.privatehand.com/flash/elements.html
4
Important Features of the Periodic Table Group
(Family)
- each column of elements on the periodic table
How many groups (families) are on the Periodic
Table Of Elements?
FROM TOP TO BOTTOM OR BOTTOM TO THE TOP
5
Important Features of the Periodic TablePeriod
(Row)
- each horizontal row of elements on the periodic
table
How many periods (rows) are on the Periodic
Table Of Elements?
FROM LEFT TO RIGHT OR RIGHT TO LEFT
6
Period (Row) Properties
- Seven periods on a periodic table (numbered from
the top down) - Atomic numbers and atomic masses increase as you
move from the left to the right in a period - All atoms of the elements in the same period have
the same number of orbitals/levels - All atoms of the elements in a specific period
have that respective number of orbitals/levels - Example
- Period 1 1 orbital (energy level)
- Period 2 2 orbitals
- Period 3 3 orbitals
- Etc
7
Examples of Period (Row) elements having the same
number of orbitals/levels in their atoms
In what period (row) do you think these atoms
reside?
In what period (row) do you think these atoms
reside?
8
Group (Family) Properties
- Eighteen groups on the periodic table (numbered
from left to right) - Atomic numbers and atomic masses increase as you
move from the top down in a group (family) - Atoms of elements in the same group have the same
number of electrons in the outer orbitals/levels
of their atoms (known as valence electrons) - Exceptions
- Transition elements (3-12)
- Hydrogen (could be 1 or 17)
- Helium (actually has 2 valence electrons)
- Elements in groups usually have similar physical
and chemical properties
9
Examples of Group Elements with the same of
valence electrons
How many electrons do each of these atoms have in
their outer orbital/level?
What group (family) do these elements reside in?
10
Group (Family) Names
Alkali Metals
Alkaline Earth Metals
Noble Gases
Boron Group
Nitrogen Group
Carbon Group
Oxygen Group
Halogens
Transition Metals
11
Identify the Element
Period 2 Group 14 ?
Carbon - C
Period 5 Group 2 ?
Strontium - Sr
Group 17 Period 6 ?
Astatine - At
Group 4 Period 7 ?
Rutherfordium - Rf