Atomic Particles - PowerPoint PPT Presentation
1 / 23
Title:
Atomic Particles
Description:
... discreet or quantised These quantum levels are now known as number states 4 3 2 1 0 energy 4hf 3hf 2hf 1hf 0 energy n Photoelectric ... Principle The Uncertainty ... – PowerPoint PPT presentation
Number of Views:103
Avg rating:3.0/5.0
Title: Atomic Particles
1
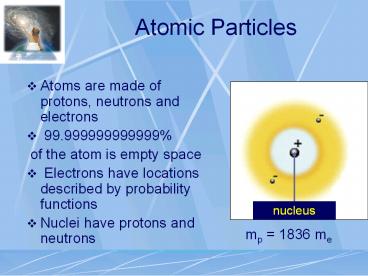
Atomic Particles
- Atoms are made of protons, neutrons and electrons
- 99.999999999999
- of the atom is empty space
- Electrons have locations described by
probability functions - Nuclei have protons and neutrons
mp 1836 me
2
Atomic sizes
- Atoms are about 10-10 m
- Nuclei are about 10-14 m
- Protons are about 10-15 m
- The size of electrons and quarks has not been
measured, but they are at least 1000 times
smaller than a proton
3
What is Light?
- Properties of light
- Reflection, Refraction
- A property of both particles and waves
- Interference and Diffraction
- Youngs double slits
- A Property of Waves Only
- Polarisation
- A Property of Waves Only
4
Classical Physics
- Light is a wave
- Youngs Double Slit Experiment
- Faradays experiments
- Maxwells equations
5
Line-Emission Spectrum
excited state
ENERGY IN
PHOTON OUT
ground state
6
Bohr Model
- e- exist only in orbits with specific amounts of
energy called energy levels - Therefore
- e- can only gain or lose certain amounts of
energy - only certain photons are produced
7
Bohr Model
- Energy of photon depends on the difference in
energy levels - Bohrs calculated energies matched the IR,
visible, and UV lines for the H atom
6
5
4
3
2
1
8
Other Elements
- Each element has a unique bright-line emission
spectrum. - Atomic Fingerprint
Helium
- Bohrs calculations only worked for hydrogen! ?
9
(No Transcript)
10
The Birth of the Quantum
- Max Planck
- The energy contained in radiation is related to
the frequency of the radiation by the
relationship - n is a positive integer called the quantum number
- f is the frequency of the oscillation
- A discreet packet of energy, later to become
known as a photon
11
Implications of Plancks Law
- The energy levels of the molecules must be
discreet - Only transitions by an amount Ehf are allowed
- The implication is that light is discreet or
quantised
energy
n
energy
4hf 3hf 2hf 1hf 0
4 3 2 1 0
These quantum levels are now known as number
states
12
Photoelectric effect
- When light strikes the cathode, electrons are
emitted - Electrons moving between the two plates
constitute a current
13
Photoelectric Effect
- Explanation
- Einstein the quanta of energy are in fact
localised particle like energy packets - Each having an energy given by hf
- Emitted electrons will have an energy given by
- Where f is known as the work function of the
material hft where ft is the threshold
frequency for the metal.
14
Louis de Broglie1892 - 1987
15
Wave Properties of Matter
- In 1923 Louis de Broglie postulated that perhaps
matter exhibits the same duality that light
exhibits - Perhaps all matter has both characteristics as
well - For photons,
- Which says that the wavelength of light is
related to its momentum - Making the same comparison for matter we find
16
Quantum Theory
- Particles act like waves?!
- The best we can do is predict theprobability
that something will happen.
Heisenberg Dirac Schrodinger
17
Schrödinger
Erwin
(1887 1961)
The task is not so much to see what no-one has
yet seen, but to think what nobody has yet
thought, about that which everybody sees.
18
Schrodingers cat
- After consultation with Einstein, Schrodinger
proposed a thought experiment in which he
highlighted the apparent inconsistencies between
the so-called Copenhagen interpretation of
Quantum Mechanics and the reality of macroscopic
measurements. - He proposed that a cat be placed in a sealed box.
The release of a poison is then subject to the
probabilistic decay of a radioactive isotope. If
the isotope decays, the poison is released. If no
decay occurs, the poison is not released. - The result is that the cat is in a superposition
of states between being dead, and being alive.
This is very unintuitive.
19
Quantum mechanics
- Wave-particle duality
- Waves and particles have interchangeable
properties - This is an example of a system with complementary
properties - The mechanics for dealing with systems when
these properties become important is called
Quantum Mechanics
20
The Uncertainty Principle
Measurement disturbes the system
21
The Uncertainty Principle
- Classical physics
- Measurement uncertainty is due to limitations of
the measurement apparatus - There is no limit in principle to how accurate a
measurement can be made - Quantum Mechanics
- There is a fundamental limit to the accuracy of a
measurement determined by the Heisenberg
uncertainty principle - If a measurement of position is made with
precision Dx and a simultaneous measurement of
linear momentum is made with precision Dp, then
the product of the two uncertainties can never be
less than h/2p
22
The Uncertainty Principle
- Virtual particles created due to the UP
23
In Search of the Higgs Boson
- Higgs boson is cosmic molasses the Holy Grail
of particle physics - Interactions with the Higgs Field are theorized
to give all the particles their masses - LHC detectors should be able to confirm or
disprove initial hints for Higgs at E115 GeV