Ch. 3: Atomic Structure - PowerPoint PPT Presentation
1 / 16
Title:
Ch. 3: Atomic Structure
Description:
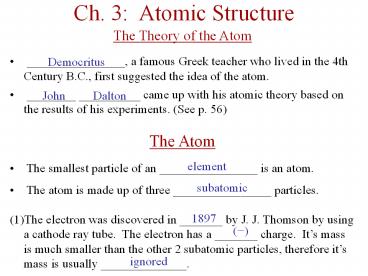
Ch. 3: Atomic Structure The Theory of the Atom _____, a famous Greek teacher who lived in the 4th Century B.C., first suggested the idea of the atom. – PowerPoint PPT presentation
Number of Views:231
Avg rating:3.0/5.0
Title: Ch. 3: Atomic Structure
1
Ch. 3 Atomic Structure
- The Theory of the Atom
- ________________, a famous Greek teacher who
lived in the 4th Century B.C., first suggested
the idea of the atom. - ________ __________ came up with his atomic
theory based on the results of his experiments.
(See p. 56) - The Atom
- The smallest particle of an ________________ is
an atom. - The atom is made up of three ________________
particles. - The electron was discovered in _______ by J. J.
Thomson by using a cathode ray tube. The
electron has a _______ charge. Its mass is much
smaller than the other 2 subatomic particles,
therefore its mass is usually ______________.
Democritus
John Dalton
element
subatomic
1897
(-)
ignored
2
Cathode Ray Tube
3
()
- (2) The proton has a ______ charge, and it was
discovered in _________ by E. Goldstein. - (3) The neutron does not have a charge. In other
words, it is ________. It was discovered in ____
by James Chadwick. The neutron has about the
same _________ as the proton. - These three particles make up all the
____________________ in the Universe! - There are other particles such as neutrinos,
positrons, and quarks, but are typically left for
2nd year chemistry courses.
1886
neutral
1932
mass
visible matter
4
- Nuclear Atomic Structure
- The atom is made up of 2 parts/sections
- (1) The ______________ --- (in the center of the
atom) - (2) The ____________ _________ --- (surrounds
the nucleus)
nucleus
electron cloud
(p n0)
e- cloud
5
- The Nucleus
- Discovered by Ernest ________________ in
________. - He shot a beam of positively charged alpha
particles, which are ___________ nuclei, at a
thin sheet of ______ _____.
Rutherford
1911
helium
gold foil
- 99.9 of the particles went right on through to
the ______________. - Some were slightly deflected. Some even
____________ ________ towards the source! - This would be like shooting a cannon ball at a
piece of tissue paper and having it bounce off.
detector
bounced back
6
Rutherfords Experiment
7
Conclusions about the Nucleus
- (1) Most of the atom is more or less _________
___________. - (2) The nucleus is very _________. (Stadium
Analogy) - (3) The nucleus is very ___________. (Large Mass
Small Volume) - (4) The nucleus is ______________ charged.
empty space
tiny
dense
positively
- Counting Subatomic Particles in an Atom
- The atomic of an element equals the number of
____________ in the nucleus. - The mass of an element equals the sum of the
_____________ and ______________ in the nucleus.
- In a neutral atom, the of protons of
______________. - To calculate the of neutrons in the nucleus,
______________ the ___________ from the
__________ .
protons
protons
neutrons
electrons
subtract
atomic
mass
8
Practice Problems
- Find the of e-, p and n0 for sodium. (mass
23) - Find the of e-, p and n0 for uranium. (mass
238) - 3) What is the atomic and mass for the
following atom? e- 15 n0 16
Atomic 11 e- p
neutrons 23-11 12
Atomic 92 e- p
neutrons 238-92 146
Atomic 15 e- p
Mass p n0 1516 31
The element is phosphorus!
9
Isotopes
- An isotope refers to atoms that have the same
of ___________, but they have a different of
___________. - Because of this, they have different _________
s (or simply, different ___________.) - Isotopes are the same element, but the atoms
weigh a different amount because of the of
______________. - Examples---gt (1) Carbon-12 Carbon-13
- (2) Chlorine-35 Chlorine-37
- (The shown after the name is the mass .)
- For each example, the elements have identical
___________ s, ( of p) but different
_________ s, ( of n0). - Another way to write the isotopes in shorthand is
as follows
protons
neutrons
mass
masses
neutrons
atomic
mass
C
Cl
12
35
6
17
mass
atomic
The top number is the ________ , and the bottom
is the __________ number. Calculating the n0
can be found by _____________ the s!
subtracting
10
Figure 3.10 Two isotopes of sodium.
11
More Practice Problems
- Find the e-, p and n0 for Xe-131.
- Find the e-, p and n0 for
- 3) Write a shorthand way to represent the
following isotope - e- 1 n0 0 p 1
Atomic 54 p e- n0 131 - 54
77
Cu
63
29
Atomic 29 p e- n0 63 - 29 34
Atomic p e- 1 mass n0 p
1 0 1
H-1 or
H
1
1
12
Ions
- An atom can gain or lose electrons to become
electrically charged. - Cation (___) charged atom created by
___________ e-s. - Cations are ______________ than the original
atom. - _____________ generally form cations.
- Anion (___) charged atom created by
_____________ e-s. - Anions are ____________ than the original atom.
- _______________ generally form anions.
- Practice Problems Count the of protons
electrons in each ion. - a) Mg2 p _____ e- ______
- b) F-1 p _____ e- ______
losing
smaller
Metals
-
gaining
larger
Nonmetals
12
10
9
10
13
Atomic Mass
- Based on the relative mass of Carbon-12 which is
exactly _______. - 1 p __ atomic mass unit (amu) 1 n0 __ amu
1e- __ amu - The atomic masses listed in the Periodic Table
are a weighted average of all the isotopes of
the element.
12
1
1
0
Weighted Average
- Practice Problems
- (1) Mrs. Smiths geometry semester grades are
calculated using a weighted average of three
category scores - Major Grades 60 of your grade
- Minor Grades 30 of your grade
- Semester Exam10 of your grade
- If a student had the following scores, what would
they receive for the semester? - Major 80 (B - )
- Minor 60 (D -)
- Semester Exam65 (D)
14
Weighted Average
- Step (1) Multiply each score by the that it
is weighted. - Step (2) Add these products up, and that is the
weighted average! - 60 x 80 48.0
- 30 x 60 18.0
- 10 x 65 6.5
- Add them up!!
- A normal average would be calculated by simply
adding the raw scores together and dividing by 3 - 80 60 65 205 3 68.3 D
72.5 (C-)
15
Average Atomic Mass
- Practice Problems
- (2) In chemistry, chlorine has 2 isotopes
- Cl-35 (75.8 abundance) Cl-37 (24.23
abundance) - What is the weighted average atomic mass of
chlorine? - 35 x 0.758 26.53
- 37 x 0.2423 8.9651
- Add them up!!!
- (3) Oxygen has 3 isotopes
- O-16 (99.76) O-17 (0.037) O-18 (0.2)
- Estimate oxygens average atomic mass.
35.4951 amu
Barely over 16.0 amu
16
Average Atomic Mass
- (4) Copper has an average atomic mass of 63.546
amu. It contains only two natural isotopes,
which are Cu-63, with an isotope mass of 62.940
and Cu-65 with an isotope mass of 64.928. What
are the percent of the two isotopes in naturally
occurring copper?
Avg. Atomic Mass (Cu-63 x Mass Cu-63)
(Cu-65 x Mass Cu-65)
Cu-63 Cu-65 100
Cu-63 Cu-65 1
OR
63.546 (Cu-63 x 62.940) (Cu-65 x 64.928)