Mass % and % Composition - PowerPoint PPT Presentation
1 / 5
Title:
Mass % and % Composition
Description:
Empirical and Molecular Formulas Empirical Formula ... Determining the Formula from Elemental Analysis: When 0.1156 g of a compound containing C, H, ... – PowerPoint PPT presentation
Number of Views:137
Avg rating:3.0/5.0
Title: Mass % and % Composition
1
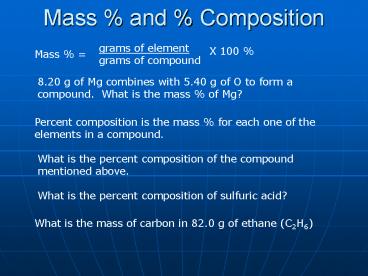
Mass and Composition
grams of element grams of compound
X 100
Mass
8.20 g of Mg combines with 5.40 g of O to form a
compound. What is the mass of Mg?
Percent composition is the mass for each one of
the elements in a compound.
What is the percent composition of the compound
mentioned above.
What is the percent composition of sulfuric acid?
What is the mass of carbon in 82.0 g of ethane
(C2H6)
2
Empirical and Molecular Formulas
Empirical Formula -
Lowest whole ratio of elements in a compound
Molecular Formula -
Actual formula of compound
The empirical formula is determined using
experimental composition data to calculate
element ratios (mole ratios).
The molecular formula is calculated from the
empirical formula and the formula mass
(mass spectrometer).
3
Empirical and Molecular Formulas
Determine the empirical formula of a compound
that is 40.0 C, 6.72 H, and 53.3 O
1 mol
3.33 mol
40.0 g C x
12.01 g
3.33 mol
6.65 mol
1 mol
6.72 g H x
3.33 mol
1.01 g
3.33 mol
1 mol
3.33 mol
53.3 g O x
16.00 g
Empirical Formula
If the molar mass of this compound is 180.1g/mol,
what is the molecular formula?
4
Empirical and Molecular Formulas
Vitamin C (ascorbic acid) is composed of 40.92
C, 4.58 H, and 54.50 O. What is the empirical
formula?
If the molar mass of ascorbic acid is 176.0
g/mol, what is the molecular formula?
5
Determining the Formula from Elemental Analysis
0.450 g of caproic acid ( a compound which
contains only H, C, and O) combusted in oxygen
gives 0.418 g H2O and 1.023 g CO2. If the molar
mass is 116.2 g/mol, what is the molecular
formula?
When 0.1156 g of a compound containing C, H, and
N is reacted with oxygen, 0.1638 g of CO2 and
0.1676 g of H2O are formed. Assuming all of the
carbon is converted to CO2 and the molar mass of
the compound is 62.0 g/mol, what is the formula
for this compound?