Percent Composition (Section 11.4) - PowerPoint PPT Presentation
1 / 11
Title:
Percent Composition (Section 11.4)
Description:
... x 100 = 38.67% Empirical vs Molecular Formulas Empirical ... Percent Composition ... To calculate percent composition Percent Composition of Water ... – PowerPoint PPT presentation
Number of Views:260
Avg rating:3.0/5.0
Title: Percent Composition (Section 11.4)
1
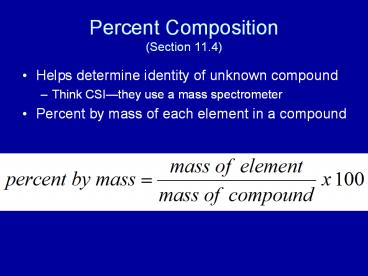
Percent Composition(Section 11.4)
- Helps determine identity of unknown compound
- Think CSIthey use a mass spectrometer
- Percent by mass of each element in a compound
2
To calculate percent composition
- H2O
- Use molar masses to calculate mass of compound
- 2 x 1.0 1 x 16.0 18.0 g/mol
- Use molar masses to calculate mass of each
individual element - H 2 x 1.0 2.0 g/mol
- O 1 x 16 16.0 g/mol
- Calculate using the formula
- H (2 / 18) x 100 11.1 Hydrogen
- O (16/18) x 100 88.9 Oxygen
- (notice to check your work 11.1 88.9 100)
3
Percent Composition of Water
- H (2 / 18) x 100 11.1
- O (16/18) x 100 88.9
- Even though there are 2 hydrogen atoms in water,
most of the mass of water comes from the 1 oxygen
atom
4
Percent Composition Practice
- KNO2 Percent composition of N
- Molar mass of N 1 x 14 14 g/mol
- Molar mass of compound 1 x 39.1 1 x 14 2 x
16 85.1 g/mol - by mass of N (14 / 85.1) x 100 16.45
5
- Calculate the percent composition
- Phosphorus in Al2(PO4)3
- P 3 x 31.0 93 g/mol
- Al2(PO4)3 2 x 27 3 x 31 12 x 16 339 g/mol
- P (93 / 339) x 100 27.43
- Chlorine in LiCl
- Cl 1 x 35.5 35.5 g/mol
- LiCl 1 x 6.9 1 x 35.5 42.4 g/mol
- Cl (35.5 / 42.4) x 100 83.73
- Calcium in CaSO4
- Ca 1 x 40.1 40.1 g/mol
- CaSO4 1 x 40.1 1 x 32.1 4 x 16 136.2
g/mol - Ca (40.1 / 136.2) x 100 29.44
6
- Calculate the percent composition
- Sodium in NaCl
- Na 1 x 23 g/mol 23 g/mol
- NaCl 1 x 23 1 x 35.5 58.5 g/mol
- Na (23 / 58.5) x 100 39.32
- Sulfur in H2SO4
- S 1 x 32.1 32.1 g/mol
- H2SO4 2 x 1 1 x 32.1 4 x 16 98.1 g/mol
- S (32.1 / 98.1) x 100 32.72
- Potassium in KNO3
- K 1 x 39.1 39.1 g/mol
- KNO3 1 x 39.1 1 x 14 3 x 16 101.1 g/mol
- K (39.1 / 101.1) x 100 38.67
7
Empirical vs Molecular Formulas
- Empirical formula formula with smallest whole
number ratio of elements - Molecular formula formula with actual number of
atoms of each element - Empirical Molecular
- C4H9 C8H18
- H2O H6O3
- We use the percent composition to calculate the
empirical and molecular formulas.
8
How to calculate empirical formula
- Use mass of elements to calculate moles
- If given percentages, assume sample is 100 grams.
Therefore, percent by mass will equal the actual
mass of element. - Pick which element has the smallest number of
moles - Divide each element by the smallest number of
moles to get ratio (these numbers become
subscripts) - Write empirical formula
9
- A blue solid is found to contain 36.43 nitrogen
and 63.16 oxygen. What is the empirical
formula? - If we assume sample is 100 grams, we have 36.43
g N and 63.16 g O. - Calculate moles of each element
- N 36.43 / 14 2.60 mol
- O 63.16 / 16 3.95 mol
- Smallest number of moles nitrogen with 2.60 mol
- Divide by smallest number of moles
- N 2.60 / 2.60 1 x 2 2
- O 3.95 / 2.60 1.5 x 2 3
- we must get whole numbers as our answerthats
why we multiplied by two - Empirical formula N2O3
10
- Determine the empirical formula for a compound
that contains 35.98 aluminum and 64.02 sulfur - If we assume sample is 100 grams, we have 35.98
g Al and 64.02 g S. - Calculate moles of each element
- Al 35.98 / 27 1.33 mol
- S 64.02 / 32.1 2.00 mol
- Smallest number of moles aluminum with 1.33 mol
- Divide by smallest number of moles
- Al 1.33 / 1.33 1 x 2 2
- S 2.00 / 1.33 1.5 x 2 3
- we must get whole numbers as our answerthats
why we multiplied by two - Empirical formula Al2S3
11
- Find the empirical formula for a substance that
contains 48.64 carbon, 8.16 hydrogen, and
43.20 oxygen - If we assume sample is 100 grams, we have 48.64
g C, 8.16 g H, and 43.20 g O. - Calculate moles of each element
- C 48.64 / 12 4.05 mol
- H 8.16 / 1 8.16 mol
- O 43.20 / 16 2.70 mol
- Smallest number of moles oxygen with 2.70 moles
- Divide by smallest number of moles
- C 4.05 / 2.70 1.5 x 2 3
- H 8.16 / 2.70 3.0 x 2 6
- O 2.70 / 2.70 1.0 x 2 2
- we must get whole numbers as our answerthats
why we multiplied by two - Empirical formula C3H6O2