Cellular Respiration - PowerPoint PPT Presentation
1 / 39
Title:
Cellular Respiration
Description:
Cellular Respiration Aerobic cell respiration NADH, made in the Krebs cycle in the matrix of the mito-chondria (its cytoplasm) carries the electrons produced when ... – PowerPoint PPT presentation
Number of Views:123
Avg rating:3.0/5.0
Title: Cellular Respiration
1
Cellular Respiration
2
Objectives
- 3.6.0 Introduction to metabolism (review)
- 3.6.1 Review enzyme kinetics and ATP
production. - 3.7.1 Define cell respiration
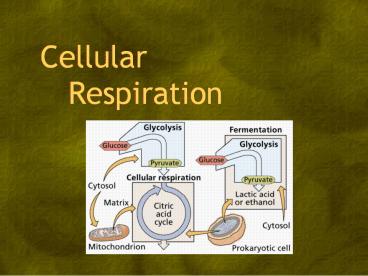
- 3.7.2 State that, in cell respiration, glucose
in the cytoplasm is broken down by glycolysis
into pyruvate, with a small yield of ATP. - 3.7.3 Explain that, during anaerobic cell
respiration, pyruvate can be converted in the
cytoplasm into lactate, or ethanol and carbon
dioxide, with no further yield of ATP.
3
Introduction to metabolism
- Energy needs of living things
- Autotrophs
- Get energy from the sun or chemicals
- Producers
- Heterotrophs
- Get energy from
consuming food - Consumers
- Herbivores
- Carnivores
- Detritivores
- Saprotrophs
- Get energy from consuming dead material
- Decomposers
4
Introduction to metabolism
- Metabolic pathways alter molecules in a series of
steps. - Enzymes selectively accelerate each step.
- Catabolic pathways release energy by breaking
down complex molecules to simpler compounds. - Anabolic pathways consume energy to build
complicated molecules from simpler compounds.
- Metabolism is the sum of chemical reactions in a
body. - Metabolic pathways alter molecules in a series of
steps. - Catabolic pathways release energy
by breaking down complex mole-
cules to simpler compounds. - Anabolic pathways consume energy
to build complicated molecules
from simpler compounds. - Enzymes selectively accelerate
each step.
Metabolic pathway
5
Introduction to metabolism
- Organisms transform energy.
- Energy is the capacity to do work - to move
matter against opposing forces. Energy is also
used to rearrange matter. - Kinetic energy is the energy of motion - ex
photons, heat. - Potential energy is the energy matter
possesses because of its location or
structure. - Chemical energy is a form of
potential energy in molecules
because of the arrangement of
atoms.
ATP
6
Introduction to metabolism
- Energy can be converted from one form to another.
- Ex as a boy climbs a ladder to the top of the
slide he is converting his kinetic energy to
potential energy. - As he slides down, the potential energy is
converted back to kinetic energy. - It was the potential energy in the food
he had eaten earlier that provided
the energy that permitted him to climb up
initially.
7
Introduction to metabolism
- Cellular respiration and
other catabolic pathways
unleash energy stored in
sugar and other complex
molecules, which were
created during photosyn-
thesis, an anabolic path- way. - CO2 H2O ? C6H12O6 O2
Anabolism
Photosynthesis ? ? ?
Catabolism
??? Respiration
8
Introduction to metabolism
- Anabolic reactions (building molecules) are
endergonic (or endothermic) ones that absorb
energy. - Ex the overall reaction of photosynthesis
- 6CO2 6H2O ? C6H12O6 6O2
- Through this reaction, energy from the sun
has been put into the chemical bonds
of a sugar molecule. The sugar has
more energy than the CO2 and H2O.
9
Introduction to metabolism
- Catabolic reactions (breaking molecules) are
exergonic (or exothermic) ones that release
energy. - Ex the overall reaction of cellular respiration
- C6H12O6 6O2 ? 6CO2 6H2O
- Through this reaction energy in the sugar
is been made available to do work in
the cell. The products (CO2 and H2O)
have less energy than the reactants.
10
Introduction to metabolism
- Exergonic vs. endergonic reactions
Respiration - Photosynthesis -
energy released for work energy
gained from the sun
11
Introduction to metabolism
- The energy created by respiration is used to do
work. - A cell does three main kinds of work
- Mechanical work beating of cilia, muscle
contraction - Transport work pumping substances across
membranes - Chemical work driving ender- gonic
reactions such as the synthesis of
polymers from monomers.
12
Introduction to metabolism
- In most cases, the immediate source of energy
that powers cellular work (coupling exergonic
reactions to endergonic reactions) is ATP
(adenosine triphosphate).
13
Introduction to metabolism
- Energy from respiration (burning food with O2) is
used to add a PO4- group to ADP. - When energy is needed by a cell, the PO4- group
is removed, and the energy is released. - The energy traveled from the sun, to the plant,
to the animal.
Exergonic ? ? Endergonic
14
Enzyme review
- Most chemical reactions do not occur
spontaneously in our bodies at 98.6o F were
too cold. - Enzymes are proteins that assist our metabolism.
- Substrates are held in the active site by weak
hydrogen bonds and ionic bonds.
Within the active site, chemical bonds are
stressed, and ATP provides the little energy
needed to start the chemical reaction.
15
Enzyme kinetics
- An enzyme is a catalytic protein.
- A catalyst is a chemical agent that changes the
rate of a reaction without being consumed by the
reaction. - Enzymes speed up metabolic reactions by lowering
energy barriers. - Ex In a match head, S O2 ?
SO2 energy, but the reaction is
not spontaneous friction
must be applied to give
some initial energy for
combustion.
friction
In a match head S O2 ? SO2 energy
16
What is cell respiration?
- Cell respiration is the controlled release of
energy from organic compounds in cells to form
ATP. - It encompasses different reactions under
different circumstances. - Anaerobic respiration no oxygen
- Glycolysis
- Fermentation
- Aerobic respiration with oxygen
- Citric acid cycle
17
Glycolysis
- Glycolysis (Greek sugar destruction) is the
first step in cell respiration. - An ancient process - occurs in all cells on
Earth. - Takes place in the cytoplasm. ?
- Does not require oxygen.
Remember only eukaryotic cells have
mitochondria.
18
Glycolysis
- Glucose is broken down into pyruvate.
- Yields a small amount of ATP only 2 molecules.
2 ATP must be used to activate the glucose
then 4 ATP are pro- duced enough to power
only a small cell. BUT without NAD, the
pathway stops.
19
Fermentations
- Fermentation allows NAD to be
regenerated, which
allows glycolysis to
continue. - Two anaerobic pathways
- Alcoholic fermentation
- Lactic acid fermentation
Sole function of fermentation is to regenerate
NAD, but there are many side benefits.
20
Alcoholic fermentation
- Pyruvate is converted in the cytoplasm into
ethanol and CO2 no more ATP, but NAD is
regenerated. - The process is present in yeast and some
bacteria. - Humans use this process to make bread, wine,
beer. - CO2 makes bread rise.
- Ethanol forms when CO2 is removed from pyruvate.
- Also important now as a bio-fuel (gasoline
substitute).
21
Alcoholic fermentation
- Yeast are critical for bread, beer,
and wine production.
Beer production line
Winery fermenters
22
Alcoholic fermentation
Production of bio-fuels Ex from starch
in corn seeds
23
Lactic acid fermentation
- Muscle cells switch from aerobic to lactic acid
ferment-ation so ATP is still produced when O2 is
scarce. - Ex athletes such as those running a marathon.
- The NAD must be regenerated to make more ATP.
- The waste product, lactate, causes muscle
fatigue, but ultimately it is converted back
to pyruvate in the liver.
24
Lactic acid fermentation
- Pyruvate is reduced directly by NADH to lactic
acid. - Lactic acid fermentation by some fungi and
bacteria is used to make cheese yogurt.
The bite of these products is due to the
lactic acid.
25
Aerobic Cell Respiration
26
Objectives
- 3.7.4 Explain that, during aerobic cell
respiration, pyru- vate can be broken down in
the mitochondrion into CO2 and H2O with a large
yield of ATP. - C.3.3 Draw and label a diagram of a
mitochondrion ex- plain the relationship
between its structure and its function. - C.3.7 Analyze data relating to respiration.
27
Aerobic cell respiration
- Remember glycolysis is the first step in both
aerobic and anaerobic respiration. - Its an ancient process (gt3 byo),
- Its found in all cells (cytoplasm),
- It converts glucose into 2 pyru- vates with a
net production of only 2 ATP. - More than ¾ of the original energy
in glucose is still present after glycolysis. - This energy can be captured in the process of
aerobic respiration.
28
Aerobic cell respiration
- With oxygen, pyruvate can be broken down further
to yield much more energy. - In the mitochondria, pyruvate is completely
oxidized to CO2 and H2O. - There is a large yield of ATP 34 more than
glycolysis.
Most of the energy within the bonds of sugar is
made available.
29
Mitochondrial structure
Mitochondria have a double membrane membrane
ridges are called the cristae, and the soupy
space between them is called the matrix. They
also have their own DNA and ribosomes.
30
Mitochondrial structure
- Mitochondrial structure is related to its
function. - They were once free-living bacteria (the theory
of endosymbiosis). - The outer membrane is thought to be the hosts,
from the original endocytosis the inner is
bacterial. - They need a lot of membrane surface area since
this is where the enzymes for respiration
are located. - More space for more energy production.
31
Aerobic cell respiration
- The 3 stages of cell respiration
- Glycolysis occurs in the cytoplasm.
- Breaks 1 glucose into 2 molecules of pyruvate
forms 2 NADH and 2 ATP. - The Krebs cycle occurs in the mitochondrial
matrix. - Degrades pyruvate to CO2 forms 2 NADH 2 ATP.
- NADH passes electrons to the electron transport
chain on the mitochondrial membrane. - Electrons eventually combine with O2 to form
water. - In the process, 34 more ATP are produced, and
NAD is regenerated to be used in glycolysis.
32
Aerobic cell respiration
No oxygen
With oxygen
33
Aerobic cell respiration
- In the Krebs cycle pyruvic
acid from glycolysis is
degraded to 3 CO2, which
are breathed out. - Two ATP and several NADH are made through
enzyme actions from each pyruvate
34
Aerobic cell respiration
- NADH, made in the Krebs cycle in the matrix of
the mito-chondria (its cytoplasm) carries the
electrons produced when pyruvate is broken down
into CO2 to the inner mitochondrial membranes
(the cristae). - The electrons are passed from one molecule to
another and give up some energy at each step. - This energy is used to pump hydrogen (H) across
the membrane, building up a high concentration
inside.
35
Aerobic cell respiration
- NADH, made in the Krebs cycle in the matrix of
the mito-chondria (its cytoplasm) carries the
electrons to the inner mitochondrial membranes
(the cristae). - The H can only exit by diffusion through a
protein called ATP synthase. - The protein is like a turbine in a dam the H
spin the protein and ADP P ? ATP.
36
Aerobic cell respiration
- The electron transfer chain
- Energy in the NADH (the electrons from glucose)
pump H into the cristae, building up a
thousand-fold concentration difference. - These diffuse out through ATP synthase making
ATP. - Aerobic respiration yields 38 ATP vs. 2 from
glycolysis alone.
The electrons eventually get picked up by
oxygen, hydrogens follow, making H2O (water).
37
Respiration poisons
- Some poisons interrupt cell respiration.
- Cyanide decouples electron transport electrons
cant reach oxygen and back-up. No NAD is
available for glycolysis, and creatures run out
of ATP and die.
38
An Analysis of respiration data
- Biosphere 2, an enormous greenhouse built in the
Arizona desert, has been used to study 5
different ecosystems. It is a closed system, so
measurements can be made under controlled
conditions. The effects of different factors,
including changes in CO2 concentration in the
greenhouse, were studied. The data shown below
were collected over the course of 1 day in
January. - Identify the time of day when the sun rose.
- Identify the time of minimal CO2 concentration.
- What was the CO2 concentration at that time?
39
An Analysis of respiration data
- Determine the maximum difference in CO2 conc.
over the 24-hr period. - What is the relationship between CO2
concentration and light intensity? - Suggest reasons for changes in CO2 conc. during
the 24-hr period.