Chap. 3 Problem 1 - PowerPoint PPT Presentation
Title:
Chap. 3 Problem 1
Description:
The hydrophobic effect also is generally important in the folding of protein structure elements. ... its aggregation and targeting to the proteasome for degradation ... – PowerPoint PPT presentation
Number of Views:97
Avg rating:3.0/5.0
Title: Chap. 3 Problem 1
1
Chap. 3 Problem 1
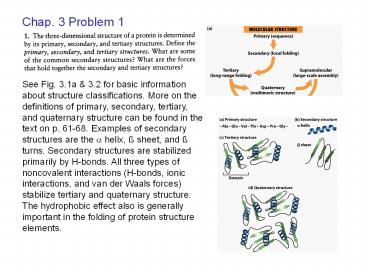
See Fig. 3.1a 3.2 for basic information about
structure classifications. More on the
definitions of primary, secondary, tertiary, and
quaternary structure can be found in the text on
p. 61-68. Examples of secondary structures are
the ? helix, ß sheet, and ß turns. Secondary
structures are stabilized primarily by H-bonds.
All three types of noncovalent interactions
(H-bonds, ionic interactions, and van der Waals
forces) stabilize tertiary and quaternary
structure. The hydrophobic effect also is
generally important in the folding of protein
structure elements.
2
Chap. 3 Problem 2
Molecular chaperones and chaperonins play
important roles in the folding of newly
synthesized proteins in cells. By binding to an
unfolded protein and promoting folding, its
aggregation and targeting to the proteasome for
degradation are prevented. Although many proteins
can fold spontaneously, chaperones reduce the
time required for folding and decrease the
likelihood that the protein will become trapped
in a partially folded state. Molecular chaperones
(e.g., Hsp70, Fig. 3.16) act as monomers to
promote folding, whereas chaperonins (e.g.,
GroELS, Fig. 3.17) are large multisubunit
machines. Both chaperonins and molecular
chaperones use ATP to catalyze folding.
Fig. 3.17a
3
Chap. 3 Problem 3
Enzymes accelerate chemical reactions by
stabilizing and thereby lowering the energy of
the transition state (Fig. 3.20). The active site
is where the substrate binds and the chemical
reaction occurs. The turnover number (kcat) is
the rate constant for the reaction. It is
equivalent to the number of substrate molecules
that can be converted to product at a single
active site per second. The Km is a reflection of
an enzymes affinity for the substrate and is
mathematically equal to the substrate
concentration at which the reaction rate is 1/2
Vmax (Fig. 3.22b, below). The lower the Km, the
higher the affinity of the enzyme for substrate.
The Vmax of an enzyme-catalyzed reaction is the
reaction rate attained when the active sites of
all enzyme molecules are bound to substrate
(saturation conditions). The rate constant is a
proportionality constant that when multiplied by
the concentration of the ES complex, gives the
reaction rate, V. V becomes equivalent to Vmax
when ES Etotal.
1/2 Vmax
4
Chap. 3 Problem 6
Many cellular proteins are degraded by a large
protein complex known as the proteasome (p.
85-88). Ubiquitin is a protein tag that targets
proteins to the proteasome for degradation (Fig.
3.29). Degradation requires polyubiquitination as
shown in the figure. The degraded protein is
cleaved to small peptides, and the ubiquitin
monomers are recycled. Proteasome inhibitors that
block degradation of tumor suppressor proteins
are being investigated as cancer therapeutics.
5
Chap. 3 Problem 7
Cooperativity refers to a change in ligand
binding affinity or enzymatic activity in a
protein or enzyme that results from
conformational changes caused by the binding of a
regulatory molecule. Binding of regulatory
molecules fine tunes the activity of the protein
often by changing its affinity for
ligand/substrate. Enzymes and binding proteins
that exhibit cooperativity have distinctive
sigmoidal shaped activity/binding curves (Fig.
3.30 hemoglobin). Protein phosphorylation and
proteolytic cleavage also are used to modulate
the function and activity of proteins and
enzymes. Ultimately these modifications change
the conformation and activity of the protein.