Chap. 19 Problem 1 - PowerPoint PPT Presentation
Title:
Chap. 19 Problem 1
Description:
The APC/C ubiquitin ligase/proteasome degrades mitotic cyclins at the end of anaphase, and this triggers telophase processes and ultimately entry into G1. – PowerPoint PPT presentation
Number of Views:60
Avg rating:3.0/5.0
Title: Chap. 19 Problem 1
1
Chap. 19 Problem 1
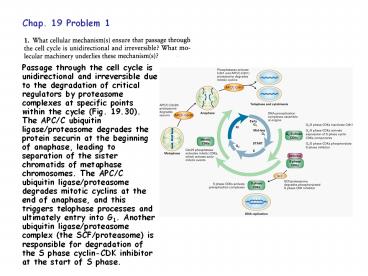
Passage through the cell cycle is unidirectional
and irreversible due to the degradation of
critical regulators by proteasome complexes at
specific points within the cycle (Fig. 19.30).
The APC/C ubiquitin ligase/proteasome degrades
the protein securin at the beginning of anaphase,
leading to separation of the sister chromatids of
metaphase chromosomes. The APC/C ubiquitin
ligase/proteasome degrades mitotic cyclins at the
end of anaphase, and this triggers telophase
processes and ultimately entry into G1. Another
ubiquitin ligase/proteasome complex (the
SCF/proteasome) is responsible for degradation of
the S phase cyclin-CDK inhibitor at the start of
S phase.
2
Chap. 19 Problem 9
At the START point in the cell cycle, cells
become committed to enter S phase regardless of
whether growth factors are present or not. In G0
phase, mitogens stimulate synthesis of G1
cyclin-CDK (cyclin D-CDK4/6) that in turn
phosphorylates the Rb protein which controls E2F
activity (Fig. 19.15b).
- Due to release from Rb control, the E2F
transcription factor induces transcription of
genes that promote entry into S phase, including
G1/S cyclin-CDKs (cyclins E/A-CDK2), S phase
cyclin-CDKs, and DNA synthesis enzymes. - Cells would no longer require mitogens for exit
of G1 if cyclin D were overexpressed. - In the absence of functional Rb, mitogens and
cyclin D would not be required for activation of
E2F. - p16 (INK4A) inhibits G1/S cyclin-CDKs. Without
p16 function, G1/S cyclin CDKs would promote
entry into S phase. - In the presence of hyperactive E2F, a number of
gene products (including E2F itself) that promote
entry of cells into S phase would be switched on.
(Refer to the first paragraph above).
3
Chap. 19 Problem 12
When S phase cyclin-CDKs are activated at the end
of G1 due to the degradation of the S phase
cyclin-CDK inhibitor, they phosphorylate two
initiation factors and MCM helicase, which leads
to unwinding of replication origins and
bidirectional DNA synthesis (Fig. 19.19). The
phosphorylated forms of the initiation factors
cannot rebind to origins preventing re-initiation
of DNA synthesis during the remainder of the cell
cycle. These factors are maintained in their
phosphorylated states by S phase and mitotic
cyclin-CDKs throughout the remainder of the cell
cycle. Only after these cyclins are degraded at
the end of mitosis can dephosphorylated
initiation factors assemble again at replication
origins.
4
Chap. 19 Problem 15
The activation of APC/C ubiquitin ligase by Cdc20
triggers the separation of sister chromatids
during anaphase (Fig. 19.27). Separation is
achieved after APC/C-mediated polyubiquitination
and proteasome degradation of the protein known
as securin. Securin normally inhibits a protease
(separase), which cleaves cohesin linkages
between sister chromatids when the inhibitor is
degraded. The protein known as Mad2 operates at
this checkpoint. Mad2 binds to kinetochores that
have not yet bound to microtubules of the mitotic
spindle. Kinetochore binding activates Mad2, and
it in turn inhibits the activity of Cdc20 which
controls the activity of the APC/C ubiquitin
ligase. This delays degradation of securin and
anaphase until all chromosomes have attached to
the spindle.
5
Chap. 19 Problem 17
Cell cycle checkpoints are points where the
status of a cells progression through the cycle
is monitored, and the cell cycle arrested if a
problem is detected. DNA damage and the
completion of DNA synthesis are monitored in G1,
S and M phases (Fig. 19.34). Potential problems
with chromosome segregation and the assembly of
the mitotic spindle are screened in M phase.
Checkpoint arrests minimize the transfer of
mutations to the next generation.