Pathway of Absorption - PowerPoint PPT Presentation
1 / 27
Title:
Pathway of Absorption
Description:
Title: No Slide Title Author: Brian Amsden Last modified by: Brian Amsden Created Date: 1/11/2001 1:41:40 AM Document presentation format: On-screen Show – PowerPoint PPT presentation
Number of Views:77
Avg rating:3.0/5.0
Title: Pathway of Absorption
1
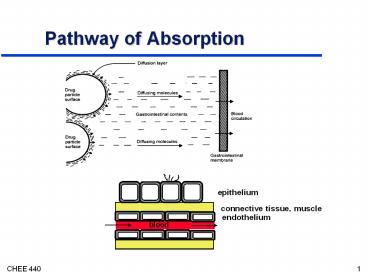
Pathway of Absorption
epithelium
connective tissue, muscle
endothelium
blood
2
Cell Membrane
3
Absorption
- Affected by
- drug chemical physical properties
- dissolution rate (solids)
- hydrophilicity/hydrophobicity
- physiological factors
- route of administration
- drug distribution
4
Solubility
- significance
- drugs must be in solution before they can be
absorbed - drugs of low aqueous solubility present
formulation problems - saturation concentration, Csat
- limit of solubility of a solute in a solvent at a
given T
5
Dissolution Rate
- important for tablets, solids
- slow dissolution rate low bioavailability
- consider a solid particle in water
stagnant water layer
Noyes-Whitney Eqn
6
Dissolution Rate
- But, surface area changes with time.
- for spherical particles
- for N particles
r radius at time t ro initial radius r
density
M mass of particles k cube-root
dissolution constant
7
Factors influencing Csat
- crystal structure polymorphism
- salt form
- pH
- solvate formation
8
Process of Dissolution
9
Crystalline Solids
- regular, ordered structure
- composed of identical repeating units - unit cell
- ex. cubic, rhombic, tetragonal
- have distinct melting pts
- strength of bonds between atoms, molecules
determines - geometry of unit cell
- Tf,
10
Crystalline Solids
- Electrostatic, Covalent Bonds
- ex. NaCl, graphite (C4)
- strong bonds - cubic unit cell
- hi Tf, hi (eg. Tf 801C for NaCl)
- stable structure
- hard, brittle
11
Crystalline Solids
- Van der Waals, H-bonds
- ex. organic compounds
- weak bonds
- low Tf, low (ex. Tf 238C for
caffeine) - soft materials
- metastable structures
12
Polymorphism
- molecule can crystallize into more than one
crystal structure - metastable form transforms to stable form over
time - usually nonreversible process - monotropic
polymorphism - many polymorphic forms possible
- progesterone - 5
- nicotinamide - 4
- dissolution rate changes with polymorphic form
13
Amorphism
- no crystal structure
- no distinct Tf
- supercooled liquids - subdued molecular motion
- flow under an applied pressure
- generally easier to dissolve
14
Crystal Hydrates
- solvent trapped when compound crystallizes -
solvates - solvent is water - hydrates
- no water - anhydrate
- solvent-compound interactions
- H2O further stabilizes lattice - polymorphic
solvates - H2O occupies void spaces - pseudopolymorphic
solvates
15
- anhydrate has higher Tf, generally dissolves
faster
16
- Significance
- incorporation of H2O affects bioabsorption rate
and ? bioactivity
17
Drug Salt Form
- salt solubility depends on nature of counter-ion
18
Slightly Soluble Electrolytes
- ex. Al(OH)3, Ca2CO3, ZnO
- AgCl(s) ? Ag(L) Cl-(L)
- Ksp Ag Cl- 1.25(10-10) at 25C
- Al(OH)3 ? Al3(L) 3OH-(L)
- Ksp Al3 OH-3 7.7(10-13) at 25C
- beware of common ion effect (salting-out)
19
pH and solubility
- weakly acidic drug
- pHp ? the pH below which the drug precipitates
from solution - weakly basic drug
- pHp ? the pH above which the drug precipitates
from solution
20
Other solubility issues
- cosolvents
- solvents which, when combined, increase the
solubility of a given compound - ex. phenobarbital in water has a solubility of
0.1g/100 ml, in alcohol 1 g in 10 ml, and in 20
alcohol/water 0.3 g/100 ml - combined effect of pH and cosolvent
- adding alcohol to buffered solution of weak
electrolyte increases solubility of undissociated
form - decreases pHp for a weakly acidic drug
21
distribution coefficient, K
- for absorption into cell, drug must pass through
lipid cell membrane - consider two immiscible phases (oil and water)
and a drug which is soluble in both (ex.
cyclosporine), at equilibrium.
oil
water
ideal and ideally dilute solutions
22
pH and Ko/w
- dissociated portion of drug does not dissolve in
oil phase - true distribution coefficient
- effective distribution coefficient
23
- as change pH, add common ion, HAw changes
weak acid
weak base
24
Clinical Significance of Ko/w
- prediction of absorption of drugs through various
tissues - absorption of acidic drugs from colon
- absorption of basic drugs from small intestine
25
- absorption of components into polymers
- plastic bottles
- PVC i.v. bags
- desorption of plasticizers from polymers
- PVC i.v. bags
26
Diffusion across a membrane
- skin, buccal mucosa, cell membrane
Cd
C1
C
Cr
C2
x
distance
27
Diffusion across a membrane
- when Cr ltlt Cd
- P permeability (cm/s)
- Ko/w D/x