Matter - PowerPoint PPT Presentation
Title: Matter
1
Matter
2
Page 4
3
Everything that has mass and volume is called
matter.
What is matter?
4
The Nature of Matter
Gold
Mercury
- Chemists are interested in the nature of matter
and how this is related to its atoms and
molecules.
5
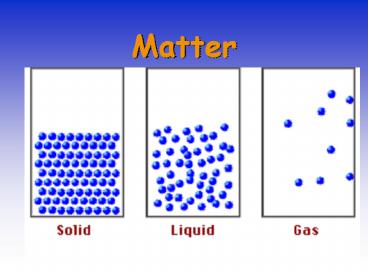
STATES OF MATTER
- _______ have rigid shape, fixed volume.
External shape can reflect the atomic and
molecular arrangement. - Reasonably well understood.
- _______ have no fixed shape and may not fill a
container completely. - Not well understood.
- _______ expand to fill their container.
- Good theoretical understanding.
6
Page 5
7
Matter Flowchart
MATTER
yes
no
Can it be physically separated?
Homogeneous Mixture (solution)
Heterogeneous Mixture
Compound
Element
8
Types of Mixtures
- Variable combination of 2 or more pure substances.
Heterogeneous visibly separate phases
Homogeneous Same throughout
9
Page 6
C
A
D
B
10
Page 7
11
(No Transcript)
12
Page 8
13
(No Transcript)
14
Page 8
Homework Pages 9 and 10
15
Page 10 11Homework 13 14
16
Kinetic Nature of Matter
- Matter consists of atoms and molecules in _____.
17
Page 12
18
Physical Properties
- What are some physical properties?
- color
- melting and boiling point
- odor
19
- Graphite layer structure of carbon atoms
reflects physical properties.
20
Physical Changes
- can be observed without changing the identity of
the substance - Some physical changes would be
- boiling of a liquid
- melting of a solid
- dissolving a solid in a liquid to give a
homogeneous mixture a SOLUTION.
21
Chemical Properties and Chemical Change
- Burning hydrogen (H2) in oxygen (O2) gives H2O.
22
Chemical Properties and Chemical Change
- Burning hydrogen (H2) in oxygen (O2) gives H2O.
- Chemical change or chemical reaction
transformation of one or more atoms or molecules
into one or more different molecules.
23
Sure Signs of a Chemical Change
- Heat
- Light
- Gas Produced (not from boiling!)
- Precipitate a solid formed by mixing two
liquids together
24
Physical vs. Chemical
- physical
- chemical
- physical
- physical
- chemical
- Examples
- melting point
- flammable
- density
- magnetic
- tarnishes in air
25
Physical vs. Chemical
- Examples
- rusting iron
- dissolving in water
- burning a log
- melting ice
- grinding spices
26
- Page 15
- Homework page 16
27
Page 16
28
- How do we separate a mixture?
- Differences in properties such as
- density
- particle size
- molecular polarity
- boiling point and freezing point
- Solubility
- These differences permit physical separation
29
Three Methods
- Filtration
- Distillation
- Chromatography
30
Separation techniques
- Filtration
- Separates by Solubility
- Examples
- Sand and Water
- Precipitate and Solvent
- Coffee, (AKA-Lifes Blood)
- Purpose
- Separate Solids from Liquids
30
31
Page 17
32
Separation techniques
- Distillation
- Boiling Point
- Examples
- Petroleum Products
- Gasoline
- Propane
- Liquors
- Purpose
- Separates Liquids
32
33
Page 18
34
Separation techniques
- Chromatography
- Density and Polarity
- Examples
- DNA
- Pigments
- Purpose
- Separates Dissolved Substances
34
35
Page 19