Lecture 7a - PowerPoint PPT Presentation
Title: Lecture 7a
1
Lecture 7a
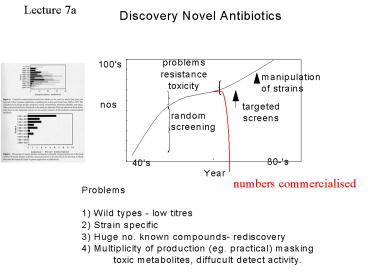
numbers commercialised
2
Costs of developing new drug and the diminishing
market
Number registered antibiotics New
drug Application NDA
Profit
Cost
NDA
Cost
1950s 1970s 2000s
3
Cost of new drug applications
4
The clinical need for new antibiotics
1. Rapid and persistent rise in antibiotic
resistance in bacterial pathogens, also in
viral infections, both in hospitals and the
community 2. Treatments required for newly
emerging bacterial infections eg Stenotrophomonas
E.coli 0157, opportunistic pathogens
Acinetobacter sp. 3. New less toxic antifungal
antibiotics needed for increased number of
systemic fungal infections 4. New antiviral
antibiotics always needed- many new strains-
avian flu 5. More broad-spectrum antibacterial
antibiotics needed to treat immunocompromised
patients in hospitals- in cases of cancer
therapy, Severe injuries, AIDS, geriatric
patients 6. Enable treatment of emerging disease
patterns in the developed world e.g. tumour
treatment, cholesterolemia, obesity, diabetes,
emphysema etc.
5
Development of new and improved antibacterial
antibiotics fighting
resistance
Rednatural poduct Blacksynthetic
chemical Underlinenovel mode action last 20 yr
6
Bacterial drug resistance and superbugs
A bacterium can migrate from one infection node
to colonise another
7
Drug Discovery Strategies
B
- Structure-based
- Rational inhibitor design based on structural
determination of target at atomic resolution - New targets from genomics
Synthesize compounds
Test activity against target
C
A
Test antimicrobial activity
Analogue Improvement
- Empirical Screening
- Natural products
- Chemical libraries
- Orthodox
- Combinatorial
- New targets from genomics
- Synthetic manipulation of existing drug
Select and develop candidate drug
Market drug
New targets in pathogens and new molecules to
combat reistsance
8
(No Transcript)
9
Combating ?-lactam resistance in pathogens
Monobactams Augmentin Clavulanic acid
Amoxycillin New form of ?-lactam
antibiotics Carbapenems gave rise to
semisynthetic imipenem
10
Commercially important antiinfectives-new
approach to drug discovery
AUGMENTIN amoxicillin-clavulanate studies,
GlaxoSmithKline
To reduce the development of drug-resistant
bacteria and maintain the effectiveness of
AUGMENTIN (amoxicillin/clavulanate potassium) and
other antibacterial drugs, AUGMENTIN should be
used only to treat or prevent infections that are
proven
Clavulanic acid is produced by the fermentation
of Streptomyces clavuligerus. It is a ß-lactam
can inactivate a variety of ß-lactamases by
blocking the active sites of these enzymes.
Clavulanic acid is particularly active against
the clinically important plasmid-mediated
ß-lactamases frequently responsible for
transferred drug resistance to penicillins and
cephalosporins
CARBAPENEMS Thienamycin discovered by Merck
broad-spectrum, good penetration bacterial cells,
high affinity for essential PBPs esp. 1a and 1b
whose inhibition causes lysis. ß.-lactamase
stable to clinically important serine
ß-lactamase. Stability of carbapenems in body was
improved by semisynthesizing imipenem still
degraded by renal dihydropeptidase so must be
co-administered with cilastatin which inhibits
peptidases. Stable against a wide variety of
ß-lactamases, including the extended-spectrum
ß-lactamases (ESBLs) and AmpC-type ß-lactamases.
Further chemical modifications lead to meropenem
and ertapenem, later shows best stability so far
in clinical use.
11
(No Transcript)
12
Example (1) Detection of novel ?-lactams using
supersensitive strains- discovery of monobactams
ß-lactam resistant ß-lactam sensitive
Strains used in plate overlay
ß-lactamase stable
Putative bioactive products A-D
zone inhibition - growth no inhibition
ß-lactamase unstable
13
Supersensitive strains for revealing new targets
- Antisense RNA for discovery of fatty acid
synthase inhibitors
Antisense RNA acts posttranscriptionally by
targeting mRNA, leading to 5 mRNA degradation.
Technology developed in order to identify
FabF/FabH target-specific cell-permeable
inhibitors by screening of natural product
extracts. Over 250,000 natural product
fermentation broths were screened and then
confirmed in biochemical assays, yielding a hit
rate of 0.1. All known natural product FabH and
FabF inhibitors, including cerulenin,
thiolactomycin, thiotetromycin, and Tu3010, were
discovered using this whole-cell mechanism-based
screening approach. Phomallenic acids, which are
new inhibitors of FabF, were also discovered.
Phomallenic acid C showed good antibacterial
activity, about 20-fold better than that of
thiolactomycin and cerulenin, against S. aureus.
It exhibited a spectrum of antibacterial activity
against clinically important pathogens including
methicillinresistant Staphylococcus aureus,
Bacillus subtilis, and Haemophilus influenzae
The active broth 6B (indicated by arrows) showed
a larger zone on the fabF AS-RNA plate, with a
diameter of 24-mm as opposed to that on the
control plate with a 9-mm diameter
14
Merck produces first-in-class antibiotic to fight
MRSA By Wai Lang Chu LATEST NEWS HEADLINE
19-May-2006 Researchers, who believe they have
discovered only the third entirely new antibiotic
developed in the last four decades, have
developed an antibiotic, which targets
microorganisms that include MRSA
Platensimycin mode of action
The fatty-acid fragment (in blue R represents a
general hydrocarbon chain) is transferred from a
sulphur atom on acyl carrier protein (ACP) to a
sulphur atom on cysteine in the FabF active site.
In response, the active site becomes more open.
b, MalonylACP substrate (in green) binds to the
acylenzyme intermediate, and loses carbon
dioxide. The fatty-acid fragment is transferred
from the active site, adding to the remains of
the malonylACP substrate, and thus extending the
chain. c, platensimycin inhibits the cycle by
binding to the acylenzyme intermediate,
preventing the addition of malonylACP substrate
thus blocking fatty-acid biosynthesis, and so
acting as antibiotic
15
Approaches to targeting vancomycin resistance
16
Antibody cleavage of depsipeptide
Chemically synthesized cleavers of depsipeptide
17
Chiosis Boneca Resensitizing resistant bacteria
to vancomycin Science 293, p1484 2001