The Periodic Table - PowerPoint PPT Presentation
Title:
The Periodic Table
Description:
The Periodic Table Elements are arranged in a way that shows a repeating, or periodic, pattern. Dmitri Mendeleev created the first periodic table of the elements in 1869. – PowerPoint PPT presentation
Number of Views:40
Avg rating:3.0/5.0
Title: The Periodic Table
1
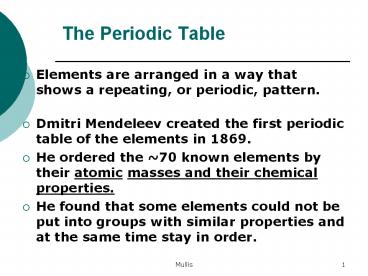
The Periodic Table
- Elements are arranged in a way that shows a
repeating, or periodic, pattern. - Dmitri Mendeleev created the first periodic table
of the elements in 1869. - He ordered the 70 known elements by their atomic
masses and their chemical properties. - He found that some elements could not be put into
groups with similar properties and at the same
time stay in order.
2
Modern Periodic Table
- Later, Henry Moseley carried on the work.
- Moseley put the elements in order of increasing
atomic NUMBER. - He found that the position of the element
corresponded to its properties. - The modern periodic table shows the position of
the element is related to - Atomic number AND
- Arrangement of electrons in its energy levels
3
Electron Shells
- 7 periods (rows)
- In general, each period represents an energy
level, or an electron shell - Move down P. table
- Principal quantum number (n) increases.
- Recall that in describing the highest energy
electron of Cl, we found that electron in this
orbital 3p5 n 3 here. - (Configuration is 1s22s22p63s23p5.)
- Atomic radius increases if same group
- Metallic character increases if same group.
4
Atomic Sizes using Periodic Table
- As we move down a group, atoms become larger.
- Larger n more shells larger radius
- As we move across a period, atoms become smaller.
- More protons more effective nuclear charge,
Zeff - More positive charge increases the attraction of
nucleus to the electrons in the outermost shell,
so the electrons are pulled in more tightly,
resulting in smaller radius
5
Ionization energy
- Ionization energy of an ion or atom is the
minimum energy required to remove an electron
from the ground state of the isolated gaseous
atom or ion. - The first ionization energy, I1 is the energy
required to remove one electron from an atom. - Na(g) ? Na(g) e-
- The 2nd ionization energy, I2, is the energy
required to remove an electron from an ion. - Na(g) ? Na2(g) e-
- Larger ionization energy, harder to remove
electron.
6
Periodic Trends in Ionization Energy
- Highest Fluorine
- Ionization energy decreases down a group.
- Easier to remove electrons that are farther from
the nucleus. - Ionization energy increases across a period.
- Zeff increases, so its harder to remove an
electron. - Exceptions Removing the 1st and 4th p electrons
7
Electron Affinity
- Electron affinity is the energy change when a
gaseous atom gains an electron to form a gaseous
ion. - Electron affinity Cl(g) e- ? Cl-(g)
- Ionization energy Cl(g) ? Cl(g) e-
Gain
Lose
8
Isoelectronic species
- Definition Has exactly the same number and
configuration of electrons - Examples Ne, Na, O2-
- Ne 10 e-
- Na 11 e- / Na 11-1 10 e-
- O 8 e- / O2- 8 2 10 e-
- Even though these have the same number of
electrons, they retain all their protons.
Therefore the sodium ion (Na) has the highest
effective nuclear charge with 11 protons
attracting its 10 electrons.
9
Ion size
- The oxide ion is isoelectronic (has exactly the
same number and configuration of electrons) with
neon, and yet O2 is bigger than Ne. Why? - In any isoelectronic series the species with the
highest nuclear charge will have the smallest
radius.
10
Metals
- Metallic character increases down a group and
from left to right across a period. - Metals are found to the left of the zig-zag line
on the periodic table. - Metal properties
- Lustrous (shiny)
- Malleable (can be shaped)
- Ductile (can be pulled into wire)
- Conduct electricity
- Metals form cations (positive ions)
- This means they lose 1-4 electrons
- Therefore, they are usually found in IONIC
compounds
11
Metal reactivity
- Which of the alkali metals would you expect to
react most violently with water? Li, Na, K, Rb - Of these four, rubidium has the lowest ionization
energy, making it the most reactive. Rubidium
reacts explosively with water.
12
Nonmetals
- Lower melting points than metals
- Diatomic molecules are nonmetals.
- The seven (7) diatomic molecules are
- Br2 I2 N2 Cl2 H2 O2 F2
- Two or more nonmetals form molecular compounds
with COVALENT bonds.
13
Trends
- See your book for full explanation.
- Closer to F more
- ELECTRONEGATIVITY
- ELECTRON AFFINITY
- IONIZATION ENERGY
- Closer to Cs more
- METALLIC CHARACTER
- ATOMIC RADIUS
- REACTIVITY