Component preparation - PowerPoint PPT Presentation
Title:
Component preparation
Description:
Title: PowerPoint Presentation Last modified by: doc Created Date: 1/1/1601 12:00:00 AM Document presentation format: On-screen Show (4:3) Other titles – PowerPoint PPT presentation
Number of Views:123
Avg rating:3.0/5.0
Title: Component preparation
1
(No Transcript)
2
Component preparation
- Principle - Differential centrifugation
- Red cells
- Packed cells
- Red cells additive
- Plasma
- Bank plasma
- Fresh frozen
- Cryo supernate
- Platelets
- Platelet rich concentrate
- Platelet rich plasma
- Cryoprecipitate
3
DEFINITIONS
- BLOOD PRODUCT Any therapeutic substance
prepared from human blood - WHOLE BLOOD Unseparated blood collected into an
approved container containing an anticoagulant
preservative solution - BLOOD COMPONENT 1. A constituent of blood ,
separated from whole blood such as - Red cell concentrate
- Plasma
- Platelet concentrates
- 2. Plasma or platelets collected by apheresis
- 3. Cryoprecipitate prepared from fresh frozen
plasma
4
Blood Components
- THE PRBC
- Storage
- - 2 6 O C
- Unit of issue
- - 1 donation ( unit or pack )
- Administration
- - ABO Rh compatible
- - Never add medication to a unit
- - Complete transfusion within 4 hrs of
commencement
1
Member
5
Dosage Administration
Dosage - 1 unit/10 kg body wt Adult dose is 4-8
units Administration - Preferably ABO Rh group
specific but not essential Other groups can be
used
6
PLATELETS
- Platelet units can be either
- Random donor units
- Apheresis units
- 1 random donor unit contains 55 x109 platelets
- 1 apheresis unit contains 240x109
7
Guidelines for Platelet Tx.
Mild - 50,000-1,00,000/µl Tx - usually not
required Moderate - 20,000-50,000/µl Tx-if
symptomatic or has to undergo surgery/trauma Sever
e - lt 20,000/µl Risk of bleeding -
high Prophylactic Tx
8
Indications for platelet transfusion
- BLEEDING due to thrombocytopaenia
- Due to platelet dysfunction
- Prevention of spontaneous bleeding with counts lt
20,000
9
IMPORTANT PRECAUTIONS
- Stored at 20-24 Degree celcius.
- Constantly agitated
- Only last for 5 days
- Infused in 30 mins
10
Fresh Frozen plasma
- Fresh frozen plasma labile nonlabile clotting
factors, albumin and immunoglobulin. Factor VIII
( 8 ) level at least 70 of normal fresh plasma
level - Storage
- - 20 C for 1 yr, - 65 C for 7 yrs.
- Before use thawed at 37 o C
11
- Fresh frozen plasma
- Indications
- - Replacement of multiple coagulation factor
deficiencies eg - Liver disease
- Anticoagulant overdose
- Depletion of coagulation factors in pts receiving
large volume transfusions - DIC (disseminated intravascular coagulation)
12
FRESH FROZEN PLASMA
- Indication
- Clinically significant deficiency of Factors
II, V, X, XI - Replacement of multiple coagulation
- factor deficiencies -
- liver disease , warfarin
treatment, - dilutional and consumption
coagulopathy - Contraindication
- Volume expansion
- Immunoglobulin replacement
- Nutritional support
- Wound healing
13
FRESH FROZEN PLASMA
- Precaution
- Acute allergic reaction are common
- Anaphylactic reaction may occur
- Hypovolemia alone is not an indication for
- use
- Dosage - Initial dose of 15 - 20 ml / kg
- Administration
- Must be ABO compatible, Rh not required
- Infuse as soon as possible after thawing
- ( within 6 hrs )
- using standard blood administration set
14
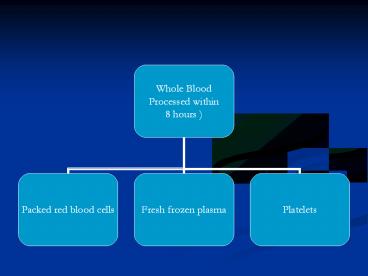
FFP
- Fresh Frozen Plasma
- Plasma collected from single donor units or by
apheresis - Frozen within 8 hours of collection
- -40o C
- Can last for a year
15
Dosage Administration for FFP
Dosage - 10-15 ml/Kg(Approx 2-3 bags for an
adult) Administration - Thawed at 37o C before
transfusion ABO compatible Group AB plasma can
be used for all patient
16
Dos and DontsIn Blood and Blood Components
17
Risk Benefit Analysis
risk gt benefit
benefit gt risk
Hb gm/dl 4 5 6 7 8 9 10 11 12
13 14
why transfuse
why not transfuse
individual patient factors decide transfusion
trigger
18
Time Limits for Infusion
Blood/ Start infusion Complete
infusion blood product Whole blood/ within 30
min. of within 4 hour red cells
removing pack (less in high
from
ambient temperature)
refrigerator Platelet
immediately within 20 min concentrates
FFP within 30 min
within 20 min
19
TRANSFUSION REACTIONS
- _at_RBCs !
- Nonhemolytic 1-5 transfusions
- Causes -Physical or chemical destruction
of - blood freezing, heating, hemolytic
drug - -solution added to blood
- -Bacterial contamination
- fever, chills, urticaria
- Slow transfusion, diphenhydramine , antipyretic
for fever - Hemolytic
- Immediate ABO incompatibility (1/ 12-33,000)
with fatality (1/ 500-800,000) - Majority are group O patients receiving type
A, B or AB blood - Complement activation, RBC lysis, free Hb (
direct Coombs Ab test)
20
Signs and Symptoms of AHTR
- Chills , fever
- Facial flushing
- Hypotension
- Renal failure
- DIC
- Chest pain
- Dyspnea
- Generalized bleeding
- Hemoglobinemia
- Hemoglobinuria
- Shock
- Nausea
- Vomitting
- Back pain
- Pain along infusion vein
21
- Anesthesia hypotension, urticaria, abnormal
bleeding - Stop infusion, blood and urine to blood bank,
coagulation screen (urine/plasma Hb, haptoglobin) - Fluid therapy and osmotic diuresis
- Alkalinization of urine (increase solubility of
Hb degradation products) - Correct bleeding, Rx. DIC
22
- _at_WBCs!
- Europe All products leukodepleted
- USA Initial FDA recommendation now reversed
pending objective data (NOT ? length of stay for
? expense) - Febrile reactions
- Recipient Ab reacts with donor Ag, stimulates
pyrogens (1-2 transfusions) - 20 - 30 of platelet transfusions
- Slow transfusion, antipyretic, meperidine for
shivering
23
- TRALI (Transfusion related acute lung injury)
- Donor Ab reacts with recipient Ag (1/ 10,000)
- noncardiogenic pulmonary edema
- Supportive therapy
24
Transfusion-related Acute Lung Injury (TRALI)
- Acute and severe type of transfusion reaction
- Symptoms and signs
- Fever
- Hypotension
- Tachypnea
- Dyspnea
- Diffuse pulmonary infiltration on X-rays
- Clinical of noncardiogenic pumonary edema
25
Transfusion-related Acute Lung Injury (TRALI)
- Therapy and Prevention
- Adequate respiratory and hemodynamic supportive
treatment - If TRALI is caused by pt. Ab ? use LPB
- If TRALI is caused by donor Ab ?no special blood
components
26
- Transfusion-associated Graft-versus-Host Disease
( TA-GVHD) - Rare immunocompromised patients
- Suggestion that more common with designated
donors - BMT, LBW neonates, Hodgkin's disease, exchange Tx
in neonates
27
Graft-versus-Host Reaction
- Signs Symptoms
- Onset 3 to 30 days after transfusion
- Clinical significant pancytopenia
- Other effects include fever, liver enzyme,
- copious watery diarrhea,
- erythematous skin erythroderma
- and
desquamation
28
- _at_Platelets!
- Alloimmunization
- 50 of repeated platelet transfusions
- Ab-dependent elimination of platelets with lack
of response - Use single donor apheresis
- Signs Symptoms
- mild ? slight fever and Hb
- severe ? platelet refractoriness with bleeding
- Post-transfusion purpura
- Recipient Ab leads to sudden destruction of
platelets 1-2 weeks after transfusion (sudden
onset) - Rare complication
29
INFECTIOUS COMPLICATIONS
- I. Viral (Hepatitis 88 of per unit viral risk)
- Hepatitis B
- Risk 1/ 200,000 due to HBsAg, antiHBc screening
(7-17 of PTH) - Per unit risk 1/63-66,000
- 0.002 residual HBV remains in negative donors
(window 2-16 weeks) - Anti-HBc testing retained as surrogate marker for
HIV
30
- NANB and Hepatitis C
- Risk now 1/ 103,000 (NEJM 96) with 2nd/ 1/
125,000 with 3rd generation HCV Ab/ HVC RNA tests
- Window 4 weeks
- 70 patients become chronic carriers, 10-20
develop cirrhosis
31
- HIV
- Current risk 1/ 450- 660,000 (95)
- With current screening (Abs to HIV I, II and p24
Ag), window 6-8 weeks (third generation ELISA
tests in Europe) - ? sero -ve window to lt 16 days
32
- HTLV I, II
- Only in cellular components (not FFP, cryo)
- Risk 1/ 641,000 (window period unknown)
- Screening for antibody I may not pick up II
- CJD (and variant CJD)
33
- II. Bacterial
- Contamination unlikely in products stored for gt
72 hours at 1-6 0 C - gram ve, gram ve bacteria
- most frequent Yersinia
enterocolitica - Produced endotoxin
- Platelets stored at room temperature for 5
days, with infection rate of 0.25 - III. Protozoal
- Trypanosoma cruzi (Chagas disease)
- Malaria
- Toxoplasmosis
- Leishmaniasis
34
Serological Testingfor Infectious markers
- HIV Ag
- Anti HIV
- HBsAg
- Anti HCV
- Test for syphilis
35
METABOLIC COMPLICATIONS
- Citrate toxicity
- Citrate (3G/ unit WB) binds Ca2 / Mg
- Metabolized liver, mobilization bone stores
- Hypocalcemia ONLY if gt 1 unit/ 5 min or hepatic
dysfunction - Hypotension more likely due to ? cardiac output/
perfusion than ? calcium (except neonates) - Worse with hypothermia/ hepatic dysfunction