Advanced Analytical Chemistry - PowerPoint PPT Presentation
1 / 17
Title:
Advanced Analytical Chemistry
Description:
Title: Put your title here Author: kelly sue rein Last modified by: Yong Cai Created Date: 7/9/2001 6:15:43 PM Document presentation format: On-screen Show – PowerPoint PPT presentation
Number of Views:110
Avg rating:3.0/5.0
Title: Advanced Analytical Chemistry
1
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/14/2006 Chapter 3 ICPMS-2
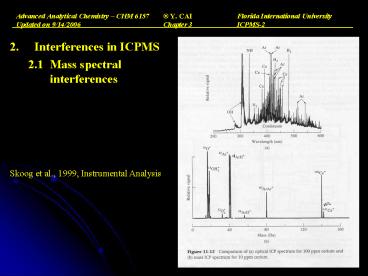
- Interferences in ICPMS
- 2.1 Mass spectral interferences
Skoog et al., 1999, Instrumental Analysis
2
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
- 2.1.1 Isobaric overlap
- Isobaric interferences are due to two elements
that have isotopes having substantially the same
mass. - Quadrupole instruments differ in mass by less
one unit.
3
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
- Generally
- Most elements in the periodic table have one
(e.g. 59Co), two (e.g. Sm, Samarium), or even
three (e.g. Sn) isotopes that are free from
isobaric overlap. - An isobaric interference occurs with the most
abundant (sad!) and thus the most sensitive
isotope, e.g. the very large peak for 40Ar
overlaps the peak for the most abundant calcium
isotope 40Ca (97) making it is necessary to use
the second most abundant isotope 44Ca (2.1). - Isotopes with odd masses are free from overlap,
while with even masses are not. - No isobaric peak interferences below 36 m/z.
- Isobaric overlaps are exactly predictable!
4
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
- 2.1.2 Polyatomic
- Polyatomic ion interferences result from
interactions between species in the plasma and
species in matrix or atmosphere. - Argon, hydrogen and oxygen are the dominant
species present in the plasma and these may
combine with each other or - With elements from the analyte matrix or
- The major elements present in the solvents or
acid used during sample preparation (e.g. N, S.
and Cl)
5
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
?? 3000000
Vandercasteele and Block 1997
6
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
Vandercasteele and Block 1997 This type of
interference is found largely at m/z values of
below 82.
7
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
Jarvis et al., 1997 Polyatomic ion peaks in
both H2O2 and HNO3 are identical to those
identified in de-ionized water and these media
are therefore considered ideal matrices. However,
the spectra in an HCl or H2SO4 matrix are more
complex.
8
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
Vandercasteele and Block, 1997
9
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
Vandercasteele and Block, 1997
10
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
Vandercasteele and Block, 1997
11
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
- Corrected for using a blanks
- Estimate the response of the interference
relative to the analyte - Reduce water entering Plasma
12
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
- 2.1.3 Refractory oxide ions
- Refractory oxide ions occur either as a result
of incomplete dissociation of the sample matrix
or from recombination in the plasma tail. - 16 (MO), 32 (MO2) or 48 (MO3) mass units above
the M peak - The relative level of oxides can be predicted
from the monoxide bond strength of the element
concerned. Those elements with the highest oxide
bond strength usually give the greatest yield of
MO ions. - Plasma operating conditions can dramatically
influence the formation of oxide ions
13
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
Jarvis et al., 1997
14
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
Jarvis et al., 1997
15
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
- 2.1.4. Doubly charged ions
- The formation of doubly charged ion in the plasma
is controlled by the second ionization energy of
the element and the condition of plasma
equilibrium. - Only those elements with a second ionization
energy lower than the first ionization energy of
Ar will undergo any significant degree of 2
formation. - The effect of 2 ions is two-fold
- Sensitivity for the singly charged species
- Spectrum interferences for others
16
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
Jarvis et al., 1997
17
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
- A note on Interferences
- Always consider and compare the relative plasma
responses of the analytes of the interest and the
interferences. Sometimes, a real experiments may
be needed.