Advanced Analytical Chemistry - PowerPoint PPT Presentation
1 / 31
Title:
Advanced Analytical Chemistry
Description:
Title: Put your title here Author: kelly sue rein Last modified by: Yong Cai Created Date: 7/9/2001 6:15:43 PM Document presentation format: On-screen Show – PowerPoint PPT presentation
Number of Views:528
Avg rating:3.0/5.0
Title: Advanced Analytical Chemistry
1
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/26/2006 Chapter 3 ICPMS-3
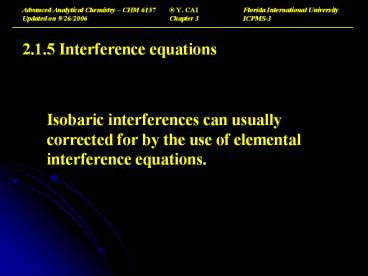
- 2.1.5 Interference equations
- Isobaric interferences can usually corrected for
by the use of elemental interference equations.
2
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/21/2006 Chapter 3 ICPMS
- Arsenic determination in a Cl matrix
- ArCl polyatomic ions formed, one of which has
the same m/z as As (75). - Cl 35 (75.77), 37 (24.23)
- As a result, quantitative analysis of arsenic can
have an error due to ArCl.
3
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/21/2006 Chapter 3 ICPMS
- ArCl is present at m/z 75 and m/z 77 in the
proportion to the isotope ratio of 35Cl 37Cl,
75.77 24.23, can be used to correct for the
interference at m/z 75. - The ArCl counts at m/z 75 are calculated based on
the m/z 77 ArCl count. By subtracting ArCl from
the count at m/z 75, the correct As concentration
can be obtained. - As (75) M (75) (75.77/24.23) x ArCl (77)
- M (75) 3.132 x ArCl (77) 1
- Where M (75) is the count number measured at m/z
75, As (75) is the count number contributed only
by arsenic at m/z 75, and ArCl (77) is the count
number contributed by polyatomic ion ArCl at m/z
77.
4
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/21/2006 Chapter 3 ICPMS
- However, as selenium has an isotope at m/z 77,
- Se 74 (0.89), 76 (9.36),
- 77 (7.63), 78 (23.78),
- 80 (49.61), 82 (8.73).
- By measuring the Se at m/z 82, the Se count at
m/z 77 can be estimated, and subtracted from the
counts at m/z 77 to calculate the counts due to
ArCl. - ArCl (77) M (77) (7.63/8.73) x Se (82)
- M (77) 0.874 x Se (82) 2
- Where M (77) is the count number measured at m/z
77 and Se (82) is the count contributed by
selenium at m/z 82.
5
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/21/2006 Chapter 3 ICPMS
- Then equation 2 can be applied to equation 1
- As (75) M (75) 3.132 x M (77) 0.874 x Se
(82) - M (75) 3.132 x M (77) 2.736 x Se
(82) 3 - So far, we have only considered ArCl and Se.
- What else?
- Kr interference at m/z 82!
- Kr 78 (0.35), 80 (2.25), 82 (11.6), 83
(11.5), 84 (57.0), and 86 (17.3).
6
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
- In some cases, Kr is found in the Argon gas
supply (mainly from bottle Ar), therefore the
signal at m/z 82 should be corrected - Se (82) M (82) (11.6/11.5) x Kr (83)
- M (82) 1.009 x Kr (83) 4
- If this equation is applied to the equation 3
- AS (75) M (75) 3.132 x M (77) 2.736 x M
(82) 1.009 x Kr (83) - M (75) 3.132 x M (77) 2.736 x M (82)
2.760 x Kr (83)
7
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
- 2.2 Matrix effects
- 2.2.1 Observation and mechanisms
- High dissolved solids
- blockage of the entrance aperture of the sampling
cone - The deposition of salts leads to a decrease in
the aperture diameter, so that the sensitivity
worsens and the signals gradually decrease as a
function of time.
8
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
- Suppression and enhancement effects
- Ionization suppression
- M M e-
- Introduction of an easily ionized element
contributes strongly to the electron density in
the plasma and therefore shifts the ionization
equilibrium so that the analyte elements are
ionized to a lesser extent. - Space charge effects
- Lighter analyte ions can be expected to suffer
more from this effect than heavier ones, and are
thus preferentially lost from the transmitted ion
beam.
9
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
- 2.2.2 Methods to correct for or overcome matrix
effects - Dilution
- Easy
- Detection limits sacrificed
- Matrix matching
- Of course, when the analyzed matrix is also
added to the standards, correction for matrix
effects is possible. This method can only be
applicable for simple matrices, e.g. metals, but
is clearly not applicable for complex matrices of
varying composition.
10
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
- Use of internal standards
- Allows correction for random fluctuations of the
signal - Allows correction for systematic variations of
the analytical signal in samples and standards
due to matrix effects - The signal for the internal standard element
should be influenced in the same way as that for
the analyte - Choose the internal standard with a mass number
as close as possible to that of the analyte
11
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
- Standard addition
- A safe method for samples of unknown composition
and thus unknown matrix effect. - Time consuming
- Chemical separation
- Allow pre-concentration of the analyte elements
- Avoidance of spectral interference.
- Isotope dilution
12
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
- Calibration and quantification
- External calibration
- Internal standard
- Standard addition
- Isotope dilution
13
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
- Isotope Dilution
- Isotope dilution is a super internal standard
addition method on the basis of isotope ratios. - Add a known amount (spike) of a stable enriched
isotope of the element considered, which has at
least two stable isotopes 1 and 2, to the sample - Measure the isotope ratio of isotopes 1 and 2 in
the Spike, the unspiked sample and finally the
spiked sample. - The concentration of the element of interest can
then be deducted from these isotopic ratios and
from the amount of spike added.
14
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
- Advantages
- Simplified chemical and physical separation
procedures - Elimination (reduction) of matrix effects
- Elimination of the effect of instrumental drift
15
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
- Theory
- In principle, any element with at least two
isotopes that can be measured is suitable for
determination by isotope dilution. The two
selected are designed 1 and 2. - Three solutions will be used
- Sample (s) Standard (t) Spiked sample (m)
16
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
- 1ns is the number of moles of isotope 1 in the
sample. - 2ns is the number of moles of isotope 2 in the
sample. - 1nt is the number of moles of isotope 1 in the
standard. - 2nt is the number of moles of isotope 2 in the
standard. - Rs is the ratio of isotope 1 to isotope 2 in the
sample solution. - Rt is the ratio of isotope 1 to isotope 2 in the
standard. - Rm is the ratio of isotope 1 to isotope 2 in the
spiked sample.
17
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
- Assuming the molecular sensitivity 1S/2S of the
MS for isotope 1 and 2 are the same, then - For the sample solution
- Rs 1ns/2ns 1
- For the standard solution
- Rt 1nt/2nt 2
18
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
- For the spiked sample solution
- Rm (1ns 1nt)/(2ns 2nt) 3
- Substitution of equations 1 and 2 into equation
3 - Rm (Rs2ns Rt2nt)/(2ns 2nt) 4
- Rearranged to
- 2ns 2nt (Rm-Rt)/(Rs-Rm) 5
- Convert the number of moles of isotope 2 in the
sample to the total number of moles of the
elements in the sample. - ns (2nt/?2)(Rm-Rt)/(Rs-Rm) 6
- ?2 is the isotopic abundance of isotope 2 in the
sample.
19
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
- The mass of the element in the sample is then
given by - Ms M(2nt/?2)(Rm-Rt)/(Rs-Rm) 7
- M is the molecular weight of the element.
20
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
- New developments in ICP/MS
- Instrumental development/improvement to
eliminate the polyatomic spectral interferences - Cool Plasma
- Collision Reaction Cell
- Dynamic Reaction Cell
- High resolution (Double-focusing analyzer)
21
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
22
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
- Cool Plasma
- The first breakthrough to reduce some of the
severe polyatomic overlaps - Use low temperature plasma to minimize the Ar and
matrix-based polyatomic species that form under
normal plasma conditions (1-1.4 KW rf power) - Cool plasma uses 500-800 KW rf power
23
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
- Unfortunately cool plasma
- Useful only for a small number of elements
- Element that form strong bond with O2 and F
cannot be decomposed because of the low plasma
energy. - Elements with high ionization potential cannot be
ionized.
24
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
- Collision Reaction Cell
Hexapoles
25
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
26
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
- Dynamic Reaction Cell
27
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
28
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
- High resolution (Double-focusing analyzer)
- (Moens and Jakubowski, Anal. Chem. 1998, 251A
256A)
29
Single Focusing Magnetic Sector
30
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS
Skoog et al, 1998
31
Advanced Analytical Chemistry CHM 6157 Y.
CAI Florida International UniversityUpdated on
9/13/2006 Chapter 3 ICPMS