Time - PowerPoint PPT Presentation
Title:
Time
Description:
Title: PowerPoint Presentation Author: Stephen Hildreth Last modified by: Stephen Hildreth Created Date: 1/13/2002 7:20:06 PM Document presentation format – PowerPoint PPT presentation
Number of Views:39
Avg rating:3.0/5.0
Title: Time
1
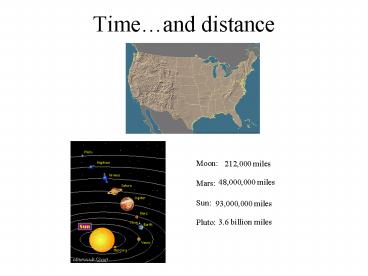
Timeand distance
Moon Mars Sun Pluto
212,000 miles
48,000,000 miles
93,000,000 miles
3.6 billion miles
2
Milky Way Galaxy
gt 100 billion suns
60,000 light years wide
Earth
Nearest star?
Alpha Centauri 4.3 light years or 25 trillion
miles
3
Galaxy Clusters
Hubble telescope estimates gt125 billion galaxies
Nearest? Andromeda 2 million light years away
For every grain of sand on Earth, there are a
million stars.
4
Geologic Time Scale
Geologic Time Scale
5
How to determine ages
How do you determine ages?
- Relative age dating. Comparing the age of one
thing relative - to something else.
2. Absolute age dating. Using analytical
techniques to determine the real age of an object.
6
Superposition
Sediments accumulating on top are younger
than those below.
7
Cross-Cutting
Rocks which cross-cut are younger than those they
cut.
8
Unconformity
Sediment deposition, followed by a period of
eroison, then more sediment deposition. End
result? A time gap.
9
Age Placing
10
Grand Canyon
11
Canyon Ages
12
Layers on Mars
13
Index Fossils
Organisms which lived only during a specific
period of time.
14
Correlation
Same rock layers, correlated over vast
distances Same rock types, ages, fossils, etc.
15
Continental Drift
Correlation was used during the development of
the continental drift theory, years ago.
16
Absolute Age Dating
Radiometic age dates were used to determine the
age of the rock layers and verify the ordering.
Relative age dating placed rock layers into a
specific order.
17
The Atom
Atom In the nucleus, there are protons () and
neutrons (neutral charge)
Surrounding the nucleus are negatively charged
electrons - used for bonding with other atoms to
form molecules
Atomic number number of protons in the nucleus
Atomic weight protons neutrons
Isotope Same number of protons, different number
of neutrons
18
Radioactivity
Some isotopes are unstable. These will decay to
stable isotopes called daughter products.
As isotopes decay, they give off subatomic
particles heat
Radiation
19
Nuclear Fuel Rods
To build a nuclear reactor, what you need is some
mildly enriched uranium. Typically, the uranium
is formed into pellets with approximately the
same diameter as a dime and a length of an inch
or so. The pellets are arranged into long rods,
and the rods are collected together into bundles.
The bundles are then typically submerged in water
inside a pressure vessel. The water acts as a
coolant. In order for the reactor to work, the
bundle, submerged in water, must be slightly
supercritical. That would mean that, left to its
own devices, the uranium would eventually
overheat and melt. To prevent this, control
rods made of a material that absorbs neutrons are
inserted into the bundle using a mechanism that
can raise or lower the control rods. Raising and
lowering the control rods allow operators to
control the rate of the nuclear reaction. When
an operator wants the uranium core to produce
more heat, the rods are raised out of the uranium
bundle. To create less heat, the rods are
lowered into the uranium bundle. The rods can
also be lowered completely into the uranium
bundle to shut the reactor down in the case of an
accident or to change the fuel.
20
Nuclear Meltdown
Explosion released 200 times more radiation than
the Hiroshima and Nagasaki bombs combined.
Chernobyl, Ukraine 1986
21
Chernobyl plume
22
Contaminated Areas
23
Sarcophagus
24
Alpha Emission
25
Beta Emission
26
Electron Capture
27
Examples
Unstable isotope
Stable daughter product
28
Half Life
Half life Over a given period of time, half the
parent isotope decays into its daughter product.
29
Examples
Uranium 238 to Lead 206 4.5 billion
years Uranium 235 to Lead 207 713 million
years Thorium 232 to Lead 208 13.9 billion
years Rubidium 87 to Strontium 87 50 billion
years Potassium 40 to Argon 40 1.5 billion years
30
Mineral Examples
Zircon
Common in granites
ZrSiO
Trace amounts of Th U
Link to radon
Radium-gtRadon-gtPolunium
Radon half life 3.8 days
EPA action level 4 picocuries per liter
31
Carbon 14 Dating
Live organisms maintain a fixed amount of
C-14 C-14 decays to N-14 after death.
Half-life 5730 yrs