Absorption Spectra - PowerPoint PPT Presentation
1 / 12
Title:
Absorption Spectra
Description:
Science is facts; just as houses are made of stone, so is science made of facts; but a pile of stones is not a house, and a collection of facts is not necessarily ... – PowerPoint PPT presentation
Number of Views:81
Avg rating:3.0/5.0
Title: Absorption Spectra
1
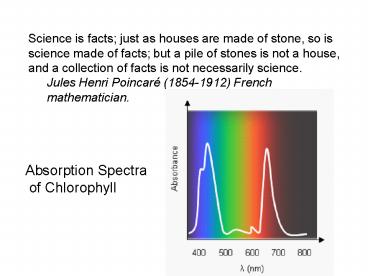
Science is facts just as houses are made of
stone, so is science made of facts but a pile of
stones is not a house, and a collection of facts
is not necessarily science. Jules Henri Poincaré
(1854-1912) French mathematician.
Absorption Spectra of Chlorophyll
2
Electromagnetic Spectrum
3
Measuring Light
- Irradiance measure of the amount of energy
falling on a flat surface (amount per unity area) - Intensity the amount of light leaving a light
source - Photon particle of light
- Quanta energy in a photon
- PPFD Photosynthetic Photon Flux Density (amount
per unit area) - PAR photosynthetically active radiation
- (350nm-700nm)
4
Measuring light
- Photometric
- Based on our eyes ability to detect
electromagnetic radiation - Units are foot-candles (fc) or lumens m-2 (lux)
5
Measuring light
- Radiometric
- Based on actual spectral distribution
- Units are
- Energy W m-2
- Number of Photons umole-m-2-s-1
Why do physiologists like photons?
6
Photosynthesis 1. Conversion of light energy
to chemical energy. 2. Short term storage of
chemical energy. 3. Long term storage of
chemical energy.
7
Basic principle of Photo-Chemistry
One photon-------One Excitation
8
Absorption Spectra of Chlorophyll
9
(No Transcript)
10
Questions to answer using figure 1.
- What is the effect of the atmosphere on direct
sunlight? - Look at the Direct Sunlight at Sea Level line.
What causes all the dips in the infra-red? - What colors (wavelengths) of light are
selectively adsorbed by clouds? - What color is sky light?
- Can you tell from this graph why leaves are
green? - What is the significance of the lack of
absorbance of infra-red radiation by vegetation?
11
(No Transcript)
12
http//www.youtube.com/profile?usergreatpacificme
diap/u/58/D6tbOBhbOZU