Rates of Reaction - PowerPoint PPT Presentation
Title:
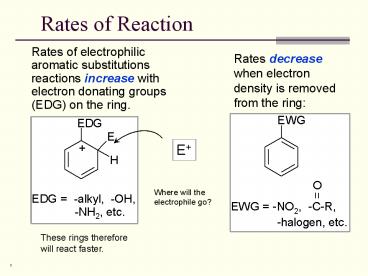
Rates of Reaction
Description:
Rates of Reaction Rates of electrophilic aromatic substitutions reactions increase with electron donating groups (EDG) on the ring. Rates decrease when electron ... – PowerPoint PPT presentation
Number of Views:132
Avg rating:3.0/5.0
Title: Rates of Reaction
1
Rates of Reaction
Rates of electrophilic aromatic substitutions
reactions increase with electron donating groups
(EDG) on the ring.
Rates decrease when electron density is removed
from the ring
E
Where will the electrophile go?
These rings therefore will react faster.
x
2
Electron Donation Ring is More Reactive
Electron donation by resonance
- Flow of pi electrons donated to ring is shown by
resonance structures.
Electron donation by induction
3
Electron Donation Ring is More Reactive
In an electrophilic reaction, where will the
electrophile find the most electron density?
- Aminobenzene is more reactive that benzene.(Rate
of electrophilic reaction is higher.) - The -NH2 substituent is an activating group.
4
Electron Withdrawl Ring is Less Reactive
Electron withdrawl by resonance
- Flow of pi electrons removed from ring is shown
by resonance structures.
Electron withdrawl by induction
Based on electronegativity
5
Electron Withdrawl Ring is Less Reactive
In this electrophilic reaction, where is the
most electron density?
- Trifluoromethylbenzene is less reactive that
benzene. - The -CF3 substituent is a deactivating group.
6
Substituent Effects in Aromatic Rings
- The more electron density a substituent donates,
the faster the rate of AES reaction. - Slowest reactions are seen with groups that
withdraw the most electron density.
7
Summary Substituent Effects
- Activating groups donate electrons to the
ring, stabilizing the carbocation intermediate - Deactivating groups withdraw electrons from
the ring, destabilizing intermediate
slower
faster
x
8
Substituent Effects
Q. How do different substituents influence the
rate of electrophilic aromatic substitution
reactions? (activate or deactivate
the ring) Q. How do different substituents
effect the orientation of the substitution
reaction? (ortho/para vs. meta
substitution)
Answer Inductive Effects Resonance Effects
x