Reaction Rates - PowerPoint PPT Presentation
Title:
Reaction Rates
Description:
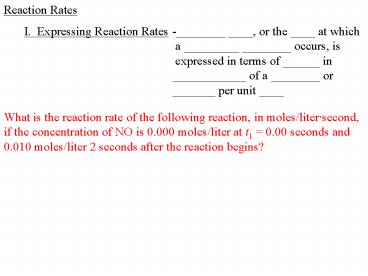
Reaction Rates I. Expressing Reaction Rates-_____ ____, or the ____ at which a _____ _____ occurs, is expressed in terms of _____ in _____ of a ... – PowerPoint PPT presentation
Number of Views:182
Avg rating:3.0/5.0
Title: Reaction Rates
1
Reaction Rates
I. Expressing Reaction Rates
-________ ____, or the ____ at which a _________
________ occurs, is expressed in terms of
______ in ____________ of a ________ or _______
per unit ____
What is the reaction rate of the following
reaction, in moles/litersecond, if the
concentration of NO is 0.000 moles/liter at t1
0.00 seconds and 0.010 moles/liter 2 seconds
after the reaction begins?
2
Reaction Rates
I. Expressing Reaction Rates
-________ ____ can be expressed as the ____ at
which a ________ is produced or the ____ at
which a ________ is consumed
What is the reaction rate of the following
reaction, in moles/litersecond, if the
concentration of C4H9Cl is 0.220M at t1 0.00
seconds and 0.100 M 4.00 seconds after the
reaction begins?
3
Reaction Rates
I. Expressing Reaction Rates
What is the reaction rate of the following
reaction, in moles/litersecond, expressed in
moles of H2 consumed, if the concentration of H2
is 0.030M at t1 0.00 seconds and 0.020M 4.00
seconds after the reaction begins?
4
Reaction Rates
I. Expressing Reaction Rates
What is the reaction rate of the following
reaction, in moles/litersecond, expressed in
moles of HCl produced, if the concentration of
HCl is 0.000M at t1 0.00 seconds and 0.020M
4.00 seconds after the reaction begins?
5
Reaction Rates
Name__________________
I. Expressing Reaction Rates
What is the reaction rate of the following
reaction, in moles/literminute, expressed in
moles of H2O2 consumed, if the concentration of
H2O2 is 2.50M at t1 0.00 minutes and 2.12M 2.00
minutes after the reaction begins?
2H2O2
1O2
2H2O
6
Reaction Rates
I. Expressing Reaction Rates
What is the reaction rate of the following
reaction, in moles/literminute, expressed in
moles of O2 produced, if the concentration of
H2O2 is 1.82M at t1 0.00 minutes and 1.48M 5.00
minutes after the reaction begins?
2H2O2
1O2
2H2O
7
Reaction Rates
II. The Collision Theory
-the _________ ______ states that, in order for
a ________ ________ to take place, the ______,
____, or _________ must _______ in order to _____
8
Reaction Rates
II. The Collision Theory
-according to the _________ ______, ___ and ___
molecules must _______ in order to _____, but in
the reaction of ______ ________ and ________
______, only a _____ _______ of the _________
produce ________. ____
9
Reaction Rates
II. The Collision Theory
10
Reaction Rates
II. The Collision Theory
11
Reaction Rates
II. The Collision Theory
-according to the _________ ______, the
_________ of ________ _________ must __ _______,
__ _______ with the correct ___________, and __
_______ with sufficient ______ to form the
_________ _______
12
Reaction Rates
II. The Collision Theory
-the minimum amount of ______ that reacting
particles must have to form the ________ _______
is called the ________ ______, or ___
-a ____ _________ ______ means that relatively
___ __________ will have sufficient ______ to
produce the _________ _______, while a ___
_________ ______ means that _____ __________
will have the required _____ to form the
__________ _______, and the _______ ____ will be
______
13
Reaction Rates
II. The Collision Theory
-once ________ ______ has been supplied to the
________, if the ________ end up lying at a
_____ ______ _____ than the _________, then
______ is ________ by the _______, and the
______ is __________
-if the ________ end up lying at a _______
______ _____ than the _________, then ______ is
________ by the _______, and the ________ is
__________
14
Reaction Rates
III. Factors Affecting Reaction Rates
A. The Nature of the Reactants
-one factor that affects the ____ of chemical
_________ is the ________ _______ of the
________
15
Reaction Rates
III. Factors Affecting Reaction Rates
B. Concentration
-when the _____________ of the _________ is
_________, reactions ______ ___
-since _________ is necessary for _________
_________ to take place, __________ the
____________ of the ________ _________ increases
the likelihood that the _________ of one
________ will _______ with the _________ of the
other _________
16
Reaction Rates
III. Factors Affecting Reaction Rates
B. Concentration
17
Reaction Rates
III. Factors Affecting Reaction Rates
C. Surface Area
-if the _______ _____ of the _____ _____ of
reactant is _________ by _________ particle
_____, the _______ ____ will ________, since the
greater ________ _____ allows the _________ of
one ________ to _______ with _____ particles of
the other ________ per unit time
18
Reaction Rates
III. Factors Affecting Reaction Rates
D. Temperature
-__________ the ___________ at which a _______
occurs _________ the _______ ____
-__________ the ___________ _________ the
average _______ _____ of the _________ that make
up a substance, causing the ________ to _______
more ___________
According to the curve of the graph, what
temperature increase, in Kelvin, doubles the rate
of reaction? _______K At what Kelvin temperature
is the relative reaction rate 25? _______K
40
35
Temperature (in K)
Relative Reaction Rate
30
25
20
Relative Reaction Rate
15
10
5
0
280
290
300
310
320
330
Temperature (in K)
19
Reaction Rates
III. Factors Affecting Reaction Rates
-__________ the ___________ also ________ the
____ of _______ by _________ the _______ of
_________ with _________ ________ ______ to
cause a _______
D. Temperature
-__________ the ___________, then, _______ the
_______ ____ by _________ the _________
_________ and the _________ ______
20
Reaction Rates
III. Factors Affecting Reaction Rates
E. Catalysts
-_________ are __________ that _______ the _____
of ________ without being _________ by the
________
Substrate (Reactant)
-________ are _________ ________ which cause
_________ to happen or happen ______ without
_______ the __________, which could ________
living things by __________ their ________
Enzyme
-________ increase ________ ____ by ________ the
_________ ______ for a _________, so that
________ that had ____________ energy before now
have _________ energy to ______
-_________ make reactions _____ likely to
_______ by ________ the __________ ______
21
Reaction Rates
III. Factors Affecting Reaction Rates
1. Hypothesis
2. Prediction
3. Gather Data
A. Safety
B. Procedure
22
Reaction Rates
III. Factors Affecting Reaction Rates
3. Gather Data
B. Procedure
23
Reaction Rates
Name_________________
III. Factors Affecting Reaction Rates
3. Gather Data
B. Procedure
Temperature (in C) Mass of Tablet (in g) Reaction Time (in s) Reaction Rate (in g/s)
4. Analyze Data
24
Reaction Rates
III. Factors Affecting Reaction Rates
4. Analyze Data
0.10
0.09
0.08
0.07
0.06
Relative Reaction Rate (in g/s)
0.05
0.04
0.03
0.02
0.01
0.00
0
10
20
30
40
50
60
70
80
90
100
Temperature (in C)
25
Reaction Rates
III. Factors Affecting Reaction Rates
4. Analyze Data
26
Reaction Rates
III. Factors Affecting Reaction Rates
5. Draw Conclusions
________________________________________________
________________________________
27
Reaction Rates
IV. Reaction Rate Laws
-when we divide the ________ in _______
____________, __________ by the _______ in
_____, ____, we get an ________ ________ _____
-chemical reactions tend to _____ _____ as
________ are _________, because in order for a
reaction to proceed, _________ must _______, and
as _________ are _________ there are ______
________ left to _______
28
Reaction Rates
IV. Reaction Rate Laws
-_____ ______ ________ the results of the
_________ _______ in terms of a ____________
___________ between the _____ of a _________
________ and the ________ _____________
-in the reaction __ ___ __, there is only ___
_________ _______ between the ________ and
________, so the _____ ____ for the reaction is
_____ __ ____, where ____ is the _____________
of the _________ __ and __ is the _____________
__________ _________ ____ ________, which
depends on _________ _________, especially the
___________
-the ________ _____, then, is _________
_____________ to the _____________
29
Reaction Rates
V. Reaction Orders
-in the reaction __ ___ __, the _____ __ ___,
and it is understood that ____ means the same
as ____, and the _________ __ is the ________
______
-the _____ ____ for the _____________ of _____
is _____ __ ______, and the ________ is said
to be _____ _____ in _____
-for _________ with _____ than _____ _______,
the _____ ____ is _____ __ ____ ____ where __
is the _______ _____ for __ and __ is the _______
_____ for __
30
Reaction Rates
V. Reaction Orders
-for the ________ that has the _____ ____
_________________, the reaction is described as
_______ _____ in ___, _____ _____ in ___, and
_____ _____ overall
-an ___________ _______ of evaluating ________
______is the _______ of _______ ______, in which
the ______________ of the _________ are _______
and the effect on the ________ _____ is observed
31
Reaction Rates
V. Reaction Orders
-the _____ ____ for this type of reaction is
_________________. From the data, you can see
that, while ____ was held constant, the
________ _____ has ________ in Trial 2 compared
to Trial 1, at the same time ____ has
________, so the ________ ______ __ must equal
__, or because ___ __, __ __
-in Trial 3 compared to Trial 2, ____ is
_______, and the _______ ____ ___________, so
the ________ ______ __ must equal __, or ___
__, and the _______ _____ ____ is ________________
_ and the _______ ________ ______ is ______
_____ _______
32
Reaction Rates
V. Reaction Orders
Given the following experimental data, use the
method of initial rates to determine the rate law
for the reaction and the overall reaction order.
33
Reaction Rates
V. Reaction Orders
Given the following experimental data, use the
method of initial rates to determine the rate law
for the reaction and the overall reaction order.
34
Reaction Rates
V. Reaction Orders
Given the following experimental data, use the
method of initial rates to determine the rate law
for the reaction, the overall reaction order, and
the value of the specific rate constant.
35
Reaction Rates
Name__________________
V. Reaction Orders
Given the following experimental data, use the
method of initial rates to determine the rate law
for the reaction, the overall reaction order, and
the value of the specific rate constant.
2OH-(aq)
2ClO2(aq)
1ClO3-
1ClO2-
1H2O(1)
Trial
Initial ClO2 (in M)
Initial Rate (in mol/Lmin)
Initial OH- (in M)
0.0500
1
0.200
6.90
0.100
2
0.200
27.6
0.100
13.8
3
0.100
36
Reaction Rates
V. Reaction Orders
Given the following experimental data, use the
method of initial rates to determine the rate law
for the reaction, the overall reaction order, and
the value of the specific rate constant.
A
B
2C
Trial
Initial A (in M)
Initial Rate (in mol/Ls)
Initial B (in M)
0.010
1
0.010
0.0060
0.020
2
0.010
0.0240
0.020
0.0960
3
0.020
37
Reaction Rates
V. Reaction Orders
Given the following experimental data, use the
method of initial rates to determine the rate law
for the reaction, the overall reaction order, and
the value of the specific rate constant.
A
B
products
Trial
Initial A (in M)
Initial Rate (in mol/Lhr)
Initial B (in M)
0.010
1
0.020
0.020
0.015
2
0.020
0.030
0.015
0.240
3
0.040
38
Reaction Rates
V. Reaction Orders
A chemical reaction involving compound A and
compound B as reactants is found to be first
order in A and second order in B. What will the
reaction rate be for Trial 2?
Trial
Initial A (in M)
Initial Rate (in mol/Ls)
Initial B (in M)
1.0
1
0.20
0.10
2.0
2
0.60
?
39
Reaction Rates
V. Reaction Orders
-most _________ _________ obey ____ of ______
_____ ______ _____ _____, _____ _____, or
_______ _____, and the ______ of the _________
_____ ________, __, vary with the ______ of the
________
-if a ________ with ____ or _____ _________ was
______________ __________ to be _____ ______
_______, the _____ _____ would be _____________
or ________________
-in ______ ______ reactions, the _____ _____ is
_________, and the ______ of __ are ________
40
Reaction Rates
V. Reaction Orders
Initial Rate (in mol/Ls)
Trial
Initial A (in M)
1
2
3
41
Reaction Rates
V. Reaction Orders
-plot the data from the table on the graph below
10.00
9.00
8.00
7.00
6.00
Reaction Rate x 10-11 (in mol/Ls)
5.00
4.00
3.00
2.00
1.00
0.00
1.00
2.00
3.00
4.00
5.00
6.00
7.00
8.00
9.00
10.00
0.00
Concentration A x 10-3 (in mol/L)
42
Reaction Rates
-if a ________ with ____ or _____ _________ was
______________ __________ to be _____ ______
_______, the _____ _____ would be _____________
or ________________
V. Reaction Orders
-in ______ ______ reactions, the _____ _____ is
_____________, and the ______ of __ are ____
Initial B (in M)
Initial A (in M)
Trial
Initial Rate (in mol/Ls)
1
2
3
4
43
Reaction Rates
V. Reaction Orders
-plot the data from the table on the graph below
10.00
9.00
8.00
7.00
6.00
Reaction Rate x 10-11 (in mol/Ls)
5.00
4.00
3.00
2.00
1.00
0.00
1.00
2.00
3.00
4.00
5.00
6.00
7.00
8.00
9.00
10.00
0.00
Concentration A x 10-3 (in mol/L)
44
Reaction Rates
-if a ________ with ____ or _____ _________ was
______________ __________ to be ______ ______
_______, the _____ _____ would be _____________
or ________________
V. Reaction Orders
-in ______ ______ reactions, the _____ _____ is
_______________, and the ______ of __ are _______
Initial B (in M)
Initial A (in M)
Trial
Initial Rate (in mol/Ls)
1
2
3
4
45
Reaction Rates
V. Reaction Orders
-plot the data from the table on the graph below
10.00
9.00
8.00
7.00
6.00
Reaction Rate x 10-11 (in mol/Ls)
5.00
4.00
3.00
2.00
1.00
0.00
1.00
2.00
3.00
4.00
5.00
6.00
7.00
8.00
9.00
10.00
0.00
Concentration A x 10-3 (in mol/L)
46
Reaction Rates
VI. Instantaneous Reaction Rates
-while the ________ _________ _____ gives the
________ _____ over a period of _____, the
____________ _____ shows
the ________ _____ at a
________ _____
Time, t (in s)
C4H9Cl (in M)
Average rate (in mol/Ls)
47
Reaction Rates
VI. Instantaneous Reaction Rates
-plot the data from the table on the graph below
0.10
0.09
0.08
0.07
0.06
C4H9Cl (in M)
0.05
0.04
0.03
0.02
0.01
0.00
0
100
200
300
400
500
600
700
800
900
1000
Time (in s)
48
Reaction Rates
VI. Instantaneous Reaction Rates
-as the reaction proceeds, and the rate of
_________ goes _____ as ________ of _________
are _________, the ______ of the _____ ________
to the _____ goes _____ as the ________ ____
goes _____
49
Reaction Rates
VI. Instantaneous Reaction Rates
If the following equation is first order in H2
and second order in NO with a rate constant of
2.90 x 102 L2/mol2s, what is the instantaneous
rate when NO 0.00200 M and H2 0.00400 M ?
50
Reaction Rates
VI. Instantaneous Reaction Rates
If the following equation is first order in H2
and second order in NO with a rate constant of
2.90 x 102 L2/mol2s, what is the instantaneous
rate when NO 0.00500 M and H2 0.00200 M ?
2NO
1H2
1N2O
1H2O
51
Reaction Rates
VI. Instantaneous Reaction Rates
If the following equation is first order in H2
and second order in NO with a rate constant of
2.90 x 102 L2/mol2s, what is the instantaneous
rate when NO 0.0100 M and H2 0.00125 M ?
2NO
1H2
1N2O
1H2O
52
Reaction Rates
VI. Instantaneous Reaction Rates
If the following equation is first order in H2
and second order in NO with a rate constant of
2.90 x 102 L2/mol2s, what is the instantaneous
rate when NO 0.00446 M and H2 0.00282 M ?
2NO
1H2
1N2O
1H2O
53
Reaction Rates
VI. Instantaneous Reaction Rates
If the following equation is first order in A and
second order in B with a specific rate constant
of 4.75 x 10-7 L2/mol2s, what is the
instantaneous rate when A 0.355 M and B
0.0122 M ?
A
B
products
54
Reaction Rates
VII. Reaction Mechanisms
-_____ chemical reactions consist of a ______ of
____ or _____ simpler _________
-a ________ ________ consists of ____ or _____
___________ steps
-for example, the _____________ of ______, an
_________ of ________, in the ______ _____ is a
________ ________ consisting of ______
___________ _________ in the following ________
__________
55
Reaction Rates
VII. Reaction Mechanisms
-because ____ is a _______ in the _____
__________ step and is _________ in the _____
___________ step, it technically _________ the
_____ of _________ without being _________
itself, and so is a _________ to the
_____________ of ______
-because both _____ and ___ are formed in ____
____________ step of the _______ ________ and
_________ in a __________ step, they are
___________
1Cl
1O3
1O2
1ClO
1O3
1O2
1O
1ClO
1O
1O2
1Cl
2O3
3O2
56
Reaction Rates
VII. Rate-Determining Step in a Complex
Reaction Mechanism
-the _______ elementary step in the ________
__________ of a ________ ________limits the
___________ ____ of the _______ _______, and so
is called the ______________ ____
-the elementary step with the _______
__________ ______ is the _______, and so is
the ______________ ____
Energy
Reaction progress